Chemistry Reference
In-Depth Information
(a)
O
-
O
Na
+
Na
+
O
O
O
O
S
S
O
O
O
+
N
N
+
N
Br
-
Br
-
N+
(b)
O
1-
N
Si
N
N
O
O
Gd
O
O
O
O
O
H
2
O
OH
2
O
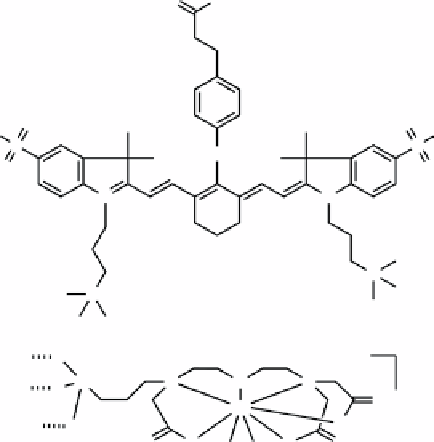
fIGure 16.18
Structures of (a) ZW800 and (b) Si-Gd(DTTA) as it might be bound to the nanoparticle surface (hashed bonds).
silica nanoparticles for trimodal imaging of tumour metastatic SNLs [74]. The ZW800 dye was modified with APTES and
incorporated into the silica matrix during the particle formation from TEOS [75, 76]. Si-DTTA (Figure 16.18b) was prepared
from bromoacetic acid and 3-(trimethoxysilylpropyl)diethylenetriamine and reacted with the silica particle surface followed
by metallation with GdCl
3
. Finally, DOTA-NHS was conjugated to the surface and metallated with
64
Cu from
64
Cu(OAc)
2
.
The relationship of 1/T
1
to Gd concentration was linear and
r
1
~ 15 mM
-1
s
-1
.
A tumour metastasis model was established by injecting 4 T1 cells into mice. The tumour metastatic SLN (T-SLN) was
compared to the normal SLN from the non-injected side of the mouse. NIRF imaging showed that the T-SLN was visible for
up to 21 days.
Ex vivo
NIRF imaging showed localisation of the probe in the liver and spleen. After two weeks, the organs
still possessed a detectable optical signal.
PET imaging showed immediate accumulation in the T-SLN. Uptake by the T-SLNs was faster than by the normal SLNs.
Between 6 and 24 h, accumulation in the liver began. MRI showed an increase in contrast in the area of the SLNs that maxi-
mised at 1 day, although contrast was observable for up to 21 days. Further, MRI tomographically shows a single layer of
T-SLNs in contrast with the 2D projection from the optical imaging results.
The authors suggest the following clinical orchestration of modalities: PET imaging can be used to first identify areas of
interest. MRI can be used to refine the anatomic localisation. Finally, optical imaging can be used to guide surgery. Overall,
the three modes of imaging give consistent results and are complementary.
16.5
upconverSIon luMIneScence
Upconversion luminescence (UCL) is the process by which continuous wave low-energy (near-infrared) light is converted
to higher energy (visible) light through multiple absorptions or energy transfers [77]. This unique luminescence process has
been observed in nanoparticles of compositions such as NaYF
4
doped with sensitiser ions such as Yb
3+
and activator ions
such as Er
3+
. UCL often has sharp emission lines, superior photostability, no autofluorescence, good tissue penetration, and
low toxicity. The topic of UCL is discussed in greater detail in Chapter 13.
16.5.1
bimodal ucl-ct nanoparticles
NaYF
4
nanoparticles were combined with 5-amino-2,4,6-triiodoisophthalic acid (AIPA) to make the first bimodal UCL/CT
contrast agent [78]. A silica shell on the UCL particles was introduced by the hydrolysis of TEOS using a standard microemul-
sion procedure. The surface was further functionalised with amines using APTES. AIPA, a standard iodine-containing CT
agent, was attached to the amines by peptide coupling. Finally, PEG groups were covalently attached using O-[2-(N-
Succinimidyloxycarbonyl)-ethyl]-O′-methylpolyethyleneglycol (SEMG, Figure 16.19). The final diameter of the particles
was found to be 54 nm. Cytotoxicity studies using MTT showed the cells to be >90% viable at the highest dose of 1000 μg/mL.