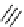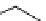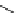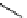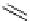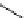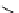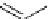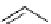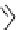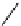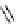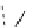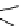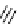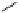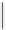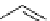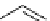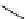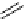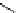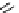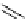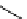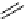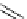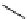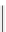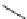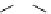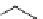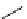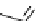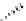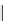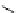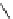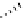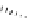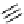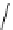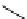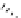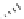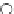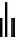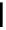Chemistry Reference
In-Depth Information
(a)
NH
N
O
N HN
N
HN
O
N
Tc
NH
NH
H
2
O
HN
Tc
O
H
2
O
H
2
O
O
Ligand 1
Ligand 2
O
O
O
Tc
O
O
O
HN
O
+
O
N
N
N
N
(c)
O
(b)
O
O
O
O
O
N
O
O
O
Tc
N
O
Tc
NH
N
S
O
R
N
N
O
O
O
R=H/CH
3
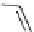
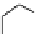
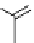
FIgure 15.7
Representative dual-modality agents that combine naturally fluorescent molecules with
99m
Tc. (a) Synthesis of acridine-
based agents. (b) aminophenylbenzothiazole-based agents. (c) a flavone-based agent.
of tumour microcalcifications (Figure 15.6B). In addition, rapid clearance of the probe from soft tissue, along with high
tumour uptake of the agent, makes it an attractive imaging agent for bone metastasis detection.
a series of dual-modality agents that combine naturally fluorescent molecules with
99m
Tc have been recently synthesised
[77-80]. Figure 15.7a shows an example reaction where a [
99m
Tc(CO)
3
(H
2
O)
3
]
+
complex is used as the precursor for the reac-
tion with a pyrazolyldiamine chelator that was functionalised with an acridine moiety. Several other representative com-
pounds of this class are also shown in Figure 15.7b and c [77-80]. although these agents have been used for cell-based
studies, they are not ideal for
in vivo
imaging applications.
aiming toward chelation of Re and Tc, single amino acid chelates (SaaCs) based on lysine derivatives have been devel-
oped [81, 82]. The construction of tridentate SaaC ligands containing two pyridine/quinoline and one tertiary amine groups
as donors was achieved by modifying the ε-NH
2
group of lysine with two pyridine/quinoline units, which readily reacts with
Re/Tc to give the [M(CO)
3
(ligand)]
+
complex in high yield. One of the major advantages of these SaaCs is their easy incor-
poration into peptide sequences by solid phase peptide synthesis. although still in the preliminary stage with no animal
studies reported, several reports exist in the literature regarding the development of potential dual-modality SPECT/optical
agents using this strategy [83-85].
In one study, SaaC was employed for
99m
Tc-labelling to create a Caspase-3-sensitive SPECT/optical imaging agent [86]. The
design of this agent is similar to the activatable PET/optical agent mentioned above [49]. The fluorescent molecule rhodamine 110
was linked to both a SaaC and a DEVD peptide, which was then labelled with
99m
Tc to form the dual-modality SPECT/optical
imaging probe. Being nonfluorescent in the bonded state, rhodamine 110 can be activated in the presence of Caspase-3, which
cleaves the DEVD sequence. after
in vitro
validation in various cell types using fluorescence techniques,
in vivo
imaging in mice
revealed significantly higher uptake of the radiolabel in apoptosis-induced liver than in normal liver [86]. This study represented
the first demonstration of dual-modality SPECT/optical imaging of enzymatic activity using a single imaging probe. The use of
NIRF dyes is expected to give better
in vivo
performance in future studies because of better tissue penetration of NIR light.
15.3.2
peptide-Based agents
as previously discussed in PET/optical agents, several RgD peptides have also been reported for SPECT/optical imaging.
The cyclic peptide c(RgDfK) was labelled with both
111
In (through DTPa) and the NIRF dye 800CW for gamma scintig-
raphy and continuous-wave imaging of integrin α
v
β
3
-positive tumours xenografts, respectively [87]. Twenty-four hours after
administration of the dual-modality agent at a dose equivalent to 90 μCi of
111
In and 5 nmol of 800CW, whole-body gamma
scintigraphy and optical imaging was conducted. It was found that the target-to-background ratios of nuclear and optical
imaging were similar for surface regions of interest, consistent with the origin of gamma and NIRF signal from a common
targeted peptide. Furthermore, an analysis of signal-to-noise ratio versus contrast showed greater sensitivity of NIRF over
nuclear imaging for subcutaneous tumour targets, while gamma scintigraphy allowed for more sensitive detection of deeper