Chemistry Reference
In-Depth Information
(a)
(c)
4
F
9/2
→
4
I
15/2
25% Yb
40% Yb
60% Yb
1
G
4
→
3
H
6
1.5% Er
1.2% Er
0.8% Er
0.5% Er
0.2% Er
(b)
(d)
I
420
490
560
630
700
λ
/nm
420
490
560
630
700
(e)
(f)
(g)
(h)
(i)
(j)
(k)
(l)
(m)
(n)
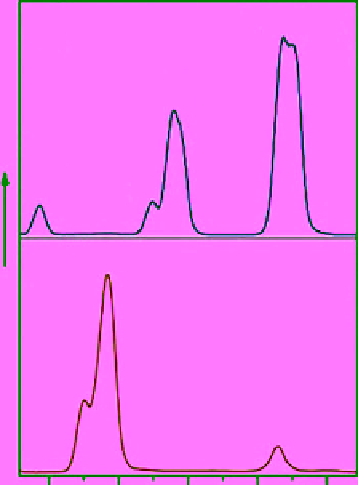
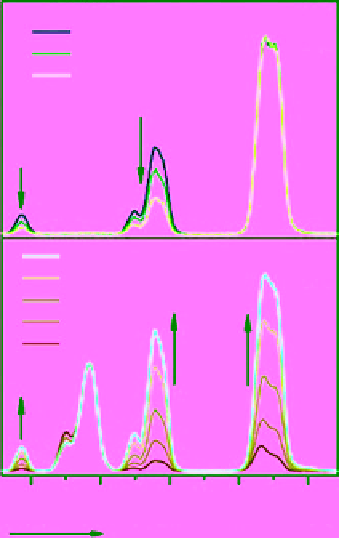
FIgure 13.1
UCL emission spectra of (a) NaYF
4
:Yb,Er (18/2 mol%), (b) NaYF
4
:Yb,Tm (20/0.2 mol%), (c) NaYF
4
:Yb,Er (25-60/2
mol%), and (d) NaYF
4
:Yb,Tm/Er (20/0.2/0.2-1.5 mol%) particles in ethanol. (e)-(n) are compiled luminescent photos showing
corresponding colloidal solutions of the samples shown in (a)-(c). Reprinted with permission from Ref. [12]. Copyright 2008 American
Chemical Society. (
See insert for colour representation of the figure.)
)
In order to obtain monodispersed Ln-UCNPs, a thermolysis method was recently developed to synthesise Ln-UCNPs.
organic solvents with high boiling points are employed to provide a high-temperature reaction medium. The concentration
of capping agents can reach a high level in these hydrophobic solvents to form a metal ion buffer. For example, Yan and
co-workers used 1-octadecence as the solvent, and oleic acid (oA) as the capping agent for the synthesis of series of rare
earth compounds [21, 22]. To obtain NaREF
4
-based UCNPs, the corresponding rare-earth trifluoroacetates can be
employed as the precursors. After heating in the solvents, rare-earth ions are coordinated by the excess oA ligands to form
a buffer system, in which the concentration of 'naked' metal ions is maintained at a stable level. The concentration of F
−
can also be controlled by adjusting the rate of the decomposition of trifluoroacetate ions. Careful control of the concentration
of rare-earth and fluoride ions results in good uniformity of the products [16, 23]. Trioctylphosphine oxide (ToPo) and
polyethylene (PEg) can be also employed as solvents, and oleylamine (om) can be used as a capping agent. A similar
method is used to synthesise various UCNPs such as NagdF
4
, NaYbF
4
, LiYF
4
, LiREF
4
, KY
3
F
10
, BagdF
5
, Y
2
o
3
, Zro
2
, and
gdoF [5].
As a variation, F
−
can be supplied as ions directly to the reaction system in the thermal decomposition protocol. Because
the concentration of F
−
used in this method is much larger than that of the 'naked' rare earth ions, the variation will be
minimal and will not affect the uniform growth of Ln-UCNPs. Using this method, Zhang and co-workers employed rare-
earth chloride NaoH and NH
4
F as rare earth, Na
+
, and F
−
sources, respectively, to synthesise hexagonal-phased NaREF
4
-