Chemistry Reference
In-Depth Information
(a)
(b)
1
2
2
h
ν
2
h
ν
3
h
ν
1
h
ν
2
1
h
ν
1
0
0
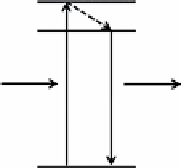
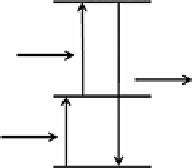
Scheme 13.1
Schematic illustration of the conventional (a) and upconversion (b) luminescence processes.
In order to satisfy the above requirements, numerous techniques have been adopted in the fabrication of UCNPs for
bioimaging applications, and these will be discussed herein.
13.2.1
general composition for ucnPs
For nano-sized upconversion materials, the most commonly used system features Yb
3+
-sensitised doping materials, with
Er
3+
, Tm
3+
, or Ho
3+
as emitters. Yb
3+
can absorb energies from the 980 nm laser excitation, and the subsequent energy transfer
upconversion (ETU) process populates the excitation state of emitters and generated upconversion emissions. In particular,
the transitions of
4
F
5/2
→
4
I
15/2
,
2
P
3/2
→
4
I
11/2
,
4
F
7/2
→
4
I
15/2
,
2
H
11/2
→
4
I
15/2
,
4
S
3/2
→
4
I
15/2
, and
4
F
9/2
→
4
I
15/2
of Er
3+
give emissions at
450, 470, 495, 525, 550, and 660 nm, respectively. The transitions of
3
P
0
→
3
F
4
,
1
D
2
→
3
H
6
,
1
g
4
→
3
H
6
,
3
F
3
→
3
H
6
, and
3
H
4
→
3
H
6
of Tm
3+
generate emissions at 353, 368, 490, 695, and 800 nm, respectively, whilst the transitions of
4
F
3
→
5
I
8
,
5
F
4
,
5
S
2
→
5
I
8
,
5
F
5
→
5
I
8
, and
5
F
4
,
5
S
2
→
5
I
7
of Ho
3+
bring out emissions at 486, 541, 647, and 751 nm, respectively. The combination of these
three doping ions can generate various colours for upconversion labelling (Figure 13.1) [5, 11, 12].
For the selection of the host matrix, rare earth-based materials are optimal because rare earth ions have similar properties,
thus enabling the effective doping of sensitiser and emitter ions in a wide concentration range. Therefore, the optically inert
rare-earth ions with full or half-full valence shells such as Y
3+
, gd
3+
, and Lu
3+
are commonly used cations in the host materials.
In selecting anions for the host materials, the final materials should have low solubility in water and other solvents, which
minimises the deliquescence effect, thus improving the stability of the materials. Until now, rare-earth halides, oxides, oxy-
sulfides, phosphates, and vanadates have been successfully used as host materials for the upconversion luminescence process.
Among them, rare-earth fluorides are considered to be one of the best choices because of their transparency in the NIR and
visible range, low phonon energies (minimising the non-radiative process), and high stability compared to other halides [13].
13.2.2
Synthetic technique
By virtue of the significant developments in nanotechnology in the past few decades, the synthesis of nano-sized upconversion
materials has been achieved by various methods (Figure 13.2). The fine-tuning of these methods can control the composition, phase,
size, and shape, as well as the surface ligands of UCNPs, which influence the upconversion properties and potential applications.
Because the commonly used host materials for Ln-UCNPs are insoluble in most solvents, co-precipitation is the easiest
method to obtain such nanoparticles by mixing the cations and anions together in the solvent. Capping ligands are usually
employed in the reaction to hinder the aggregation and control the growth of nanoparticles.
In 2004, NaYF
4
:Yb,Er/Tm nanoparticles were synthesised by Yi and co-workers via a simple co-precipitation method
[19]. NaF was used as the precipitator and ethylenediaminetetraacetic acid (EDTA) was employed as the capping agent to
control the size of the products. An annealing process promoted the cubic-to-hexagonal phase transition and enhanced the
upconversion luminescence emissions. many other upconversion nanoparticles, such as Y
2
o
3
, gd
2
o
3
, Lu
2
o
3
, gd
4
o
3
F
6
,
gd
3
ga
5
o
12
, gdoF, BaYF
5
, NagdF
4
, LuPo
4
, and YbPo
4
have also been synthesised via similar co-precipitation methods by
the careful selection of precipitators [5].
The hydro(solvo)thermal method is also based on the principle of co-precipitation. The difference is that the relatively
violent reaction conditions accelerate the crystallisation and recrystallisation rates, thus leading to kinetically stable products
with high crystallinity. Taking NaREF
4
, which is regarded as the most efficient host material for the upconversion lumines-
cence (UCL) process as an example, hexagonal-phased Ln-UCNPs will be obtained after extended hydrothermal treatment,
although cubic phase materials can be formed at the beginning of the reaction. In a common hydrothermal process, rare-earth
ions and anion sources are added to the solvent, and cubic phase precipitations will immediately form. After hydrothermal
treatment, the recrystallisation process occurs with the formation of hexagonal Ln-UCNPs. The size and shape can be fine-
tuned by controlling reaction conditions such as temperature, time, and capping agents [14, 20].