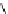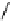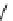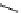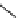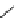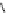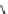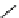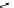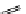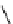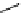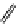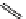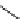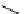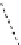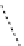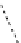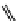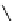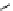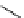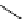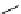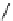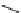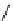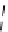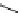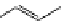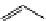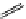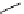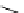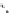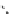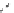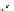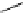Chemistry Reference
In-Depth Information
Intriguingly, the ratiometric luminescence characteristics of Eu
III
can be exploited using hyper-spectral analysis of microscopy
images to signal changes in intracellular biochemical species in real time. Complexes with an amide-linked azaxanthone
chromophore and two coordinated water molecules selectively and reversibly bind bicarbonate (for example, Scheme 12.5),
intracellularly modulated by variation in external pCO
2
; in a cellular context the complexes localise in the mitochondria of
living cells and indicate a bicarbonate concentration of 10-30 mM [65].
Whilst numerous examples of dimetallic, bis-macrocyclic Ln
III
complexes (Figure 12.25) have been reported for a variety
of studies, the nature of the bridging unit can also be used advantageously in a cellular imaging context. Wong has reported
the dimetallic Eu
III
complex of a ligand, comprising two dO3A units linked via a bent biphenyl-methylene type bridging
chromophore, that demonstrates binding to HSA (log k = 4.84) through an enhancement of sensitised (λ
ex
= 350 nm) Eu
III
emission. Altering the bridging chromophore to a linear dimethoxy-biphenyl bridge inhibited the binding ability of the com-
plex. Confocal fluorescence microscopy imaging with HeLa cells (Figure 12.25) showed that both complexes are taken up
by the cells and distributed in the cytoplasm, with the more lipophilic bent complex demonstrating much more rapid uptake
and reduced cytotoxicity (IC
50
= 3 mM) [66].
12.3.4.2 Acyclic Complexes
The proclivity of cyclen-derived Ln
III
complexes has not precluded the development of
other ligand types for the biological exploitation of Ln
III
complexes. Of the cellular imaging work conducted using Ln
III
complexes of non-macrocyclic/cyclen type ligand frameworks, the most promising have been the developments by Bünzli
of bimetallic, triple-stranded type helical complexes (discussed briefly above) of the general formula [Ln
2
L
3
], relatively high
MW probes (>2500 da) that are formally charge neutral. The bimetallic complexes can be formed through self-assembly
under physiologically mimicked conditions [67]. Within each discrete complex the two Ln
III
ions benefit from a nine-
coordinate tri-capped trigonal prismatic coordination sphere, which encapsulates the ion tightly and prevents the approach
of inner sphere water. Whilst these complexes would appear to be less water soluble than the more common (poly)amino-
carboxylate type species, their hydrophilicity can be appropriately tuned through the addition of polyoxoethylene chains to
the ligand periphery [68]. In fact, these ligands can be advantageously adorned (Figure 12.26) with a variety of substituents
(e.g., positions R
1-3
) that allow issues of solubility, optical/photophysical tuning, bioconjugation, and cell permeability to be
effectively addressed, rendering this class of species particularly attractive in an imaging context. The Eu
III
complexes benefit
from good overall quantum yields and long millisecond lifetimes in water, whilst single-photon excitation wavelengths can
be easily tuned toward 400 nm and thus biocompatibility with common 405 nm laser lines. The thermodynamic, photophysical,
and biochemical attributes of homo-bimetallic helicates have proved to be sufficient for cellular imaging applications.
For the imaging studies, both cancerous (HeLa, MCF-7, HaCat) and non-cancerous (Jurkat) cell lines have been investi-
gated. It was found that incubation concentrations could be as low as 5 μM, with uptake defined through endocytosis with
the Eu
III
complexes showing staining of the cytoplasm, where the helicates localise in lysosomes (which localise around the
nucleus) and liposomes of the ER. Co-localisation experiments with known organic dyes (such as ER-Tracker, Blue-White
dPX) suggest that the complex stains vesicles localised in the ER, rather than the golgi apparatus [69]. Egress can be very
slow and limited to around 30% over a 24 hr period, and the complexes have been shown to be non-cytotoxic. grafting PEg
chains (i.e., increasing hydrophilicity) does not appear to alter the uptake mechanism or localisation characteristics of the
O
OMe
N
O
O
O
N
O
N
N
O
O
Eu
N
N
E
u
O
N
H
N
O
N
H
2
O
MeO
O
O
O
OH
2
O
O
O
O
O
OH
2
O
O
N
N
N
O
O
Eu
N
O
Eu
N
N
O
N
H
H
N
O
O
O
H
2
O
O
O
FigUre 12.25
dimetallic Eu
III
complexes with bridging chromophores.