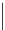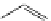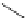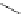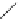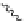Chemistry Reference
In-Depth Information
studies with gd
III
complex analogues) and excited state quenching (e.g., Stern-Volmer plots show that urate is a very effec-
tive quencher of dO3A-type complexes) phenomena.
12.3.4.1 Macrocyclic Complexes
The cyclen ligand framework has provided one of the ideal structural scaffolds upon
which to build multidentate ligands suitable for Ln
III
encapsulation, providing complexes with high kinetic and thermody-
namic stability. The reaction chemistry of the cyclen ring-nitrogens allows functional groups to be added in a stepwise
manner, thereby allowing a huge variety of ligands to be synthesised. A significant body of work has been undertaken by
Parker that investigated the cellular imaging potential of a diverse range of monometallic Ln
III
-based optical probes (visibly
emissive Eu
III
Φ up to 10% and Tb
III
Φ up to 40%) complexes), all of which are based on the cyclen ligand framework [59].
generally, each of the complexes possesses a sensitising chromophore (Figure 12.21; e.g. tetraazatriphenylene, azaxan-
thone, azathiaxanthone, pyrazolyl-azaxanthone [60], acridone [61]), which is covalently linked to the macrocyclic frame-
work and can also coordinate to the Ln
III
if desired. The remainder of the coordination sphere comprises either phosphinate,
amide, or carboxylate donors; these groups, and the associated periphery of the ligand architectures, can be designed to
control overall charge and influence lipophilicity.
The types of cell used in these studies were NIH-T3, CHO, and HeLa, and flow cytometry quantified cellular uptake.
Typical incubations were conducted at 50 μM and cell loading correlated with approximately 10
8
complexes per cell; the
dominant mechanism of uptake for these complexes is macropinocytosis (the formation of large endocytotic vesicles of
irregular shape and size). The key discovery from these far-ranging studies is that the nature and linkage of the sensitising
chromophore is the most important factor in determining cellular uptake and localisation; unlike the
d
-metal complexes
described earlier, charge, lipophilicity, and donor group substituents are not a critical factor. The nature of the aromatic
planar chromophore is often implicated in protein binding and thus, presumably, trafficking. The intracellular localisation
profile that is observed for the majority of these macrocyclic Ln
III
complexes is endosomal-lysosomal (confirmed through
co-staining experiments with LysoTracker); generally the rates of uptake and egress are fast. A smaller number of complexes
show fast uptake and slow egress and rapid shuttling between endosomal/lysosomal compartments and mitochondria
(confirmed through co-staining experiments with MitoTracker), and such behaviour does not compromise the mitochondrial
membrane potential (i.e., the complexes are nontoxic). Those complexes (Figure 12.22) that do localise in the mitochondria
for long periods of time (up to 10 hrs) demonstrate lower IC
50
values [62].
Ln
III
complexes that incorporate an
N
-coordinated azathiaxanthone sensitiser show localised preferences for protein rich
domains (ribosomes, nucleoli). Unlike the majority of the complexes, these species are characterised by slow uptake, slow
egress, and moderate toxicity (IC
50
40-90 μM), which is attributed to the specific chemical nature of the antenna (i.e., the
product of oxidative metabolism at sulphur, giving a sulfoxide or sulfone, the latter resulting in significant cytotoxicity; the
azaxanthone structural analogues were shown not to be toxic in control experiments). The known dNA intercalating ability
of the chromophore may determine the fate of the complexes, which appear only to penetrate compromised membranes,
ultimately localising in areas of chromosomal dNA. In fact, monocationic complexes incorporating two azaxanthone-
type chromophores in the 1- and 7-positions of the cyclen ring (Figure 12.23) have demonstrated selective staining of chro-
mosomal dNA in dividing cells [63]. However, it is interesting to note that although the complexes possess low intrinsic
N
N
O
N
X
N
N
O
Tetraazatriphenylene
Azaxanthone
N
N
N
O
NS
CMe
3
O
O
Pyrazoyl-azaxanthone
Azathioxanthone
FigUre 12.21
Examples of the aromatic sensitising chromophores utilised in Parker's complexes.