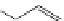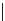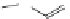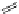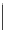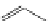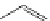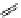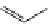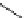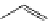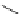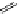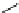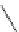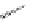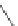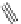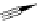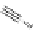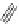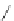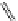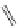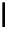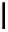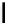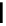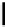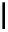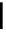Chemistry Reference
In-Depth Information
O
Et
NH
HN
N
O
Et
O
N
HN
H
S
O
N
OC
N
Re
OC
N
CO
9
FigUre 12.9
Biotin-appended rhenium complex
9
.
R
10
R=H
N
O
OC
11
R=
N
H
Re
O
OC
N
R=
12
CO
O
FigUre 12.10
Structure of complexes
10-12
.
O
NH
2
HN
PNA
(H
2
C)
3
O
N
N
O
O
Cl
Re
Re
20 μm
O
O
Cl
O
O
13
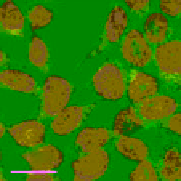
FigUre 12.11
Thymine-rich PNA appended complex
13
and cell imaging showing blue-shifted nuclear emission (Reproduced with
permission from Ref. [23]).
cyclohexyl amide-substituted complex
11
is not taken up by healthy cells, while the analogous ester
12
is taken up well by
passive diffusion [22]. In this sense, the lower lipophilicity of the rhenium core is more attractive than the highly lipophilic
bis-cyclometallated [Ir(ppy)
2
(N^N)] core, because small variations can be used to tune localisation, while it seems more diffi-
ficult to overcome the affinity of the [Ir(ppy)
2
(N^N)] unit to show nonspecific distribution throughout lipophilic regions of cells.
A single example of an unusual complex based on a pyridazine-bridged di-rhenium core
13
(Figure 12.11) appended
with a thymine PNA unit has been shown to be taken up well by cells and localise in the nucleus, directed by the PNA unit
[23]. The emission from the nucleus is blue-shifted compared to that from other cellular regions, indicative of an interaction
with dNA.
12.2.3.5 Summary of Rhenium Cell-Imaging Studies
Rhenium complexes have proven to be highly amenable to tuning of
uptake and localisation properties by making small changes to the pyridine substituent, although the photophysics are not as easy
to tune as some other classes of complex. The lower lipophilicity of the rhenium core is more attractive than the highly lipophilic
bis-cyclometallated [Ir(ppy)
2
(N^N)] core, because these small variations can be used to tune localisation, while it seems more dif-
ficult to overcome the affinity of the [Ir(ppy)
2
(N^N)] unit to show nonspecific distribution throughout lipophilic regions of cells.