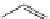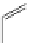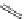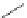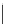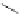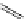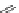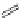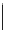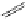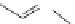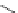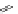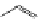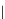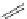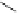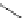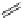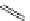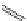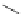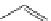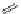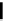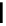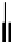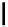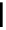Chemistry Reference
In-Depth Information
the cations [Re(bipy)(CO)
3
(L)]
+
show longer lifetimes (up to ms) and larger quantum yields (1-10%) [17]. Although the
excitation maxima are still in the UV region (~380 nm), the characteristic broad excitation bands associated with
1
MLCT
absorption mean that excitation with visible light (often a 405 nm laser line) is effective, and the large Stokes' shift (>100 nm,
emission maxima ~550 nm) allows easy detection that is conveniently free from autofluorescence.
The ease of synthesis of a range of complexes of the general formula [Re(CO)
3
(bipy)(PyX)]
+
in which bipy refers to
2,2′-bipyridine or a similar system, and PyX a substituted pyridine has allowed a systematic variation of both the photophysical
properties (by variation in the bipy unit) and the cellular behaviour (by variation in the pyridine). There are now cellular imaging
studies of over 50 complexes reported in the literature that demonstrate that variations of the bipy unit have relatively minor
effects on the uptake and localisation of the complexes (at least for the examples so far reported), whereas variations in the pyr-
idine substituents can be used to give a degree of control of the uptake and cellular localisation of the agents.
12.2.3.3 Synthesis
Typically, the synthesis of rhenium-based imaging agents follows the approach outlined in Scheme 12.2.
The lumophoric [Re(CO)
3
(N^N)] core is first prepared by the reaction of rhenium halide pentacarbonyl with the chelating
bisimine N^N. This species is then activated to ligand exchange by abstraction of the halide with silver salts, typically to gen-
erate the labile acetonitrile adduct, which is then reacted further with a substituted pyridine to give the cationic probe.
12.2.3.4 Development of Rhenium Imaging Agents
The first report of application of [Re(CO)
3
(N^N)PyX] species in cell
imaging appeared in 2007, applying a range of cationic, anionic, and neutral complexes in the imaging of parasitic flagellates
(
Spironucleus Vortens
) [18]. This preliminary study demonstrated that variations in the charge and lipophilicity of the
complexes were effective in controlling uptake and cellular localisation with wide variations observed. A series of subsequent
studies by a number of groups has shown that many complexes of this general structure are taken up well in a variety of mam-
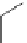
malian cells and that cellular localisation can be controlled by substitution patterns [19]. For instance, a chloromethyl-substituted
complex
8
(Figure 12.8) targets mitochondria, in line with the known propensity of chloromethyl units to react with reduced
thiols, in which mitochondria are particularly rich [20]. Biotin-appended complexes have been explored as a method of assist-
ing cell uptake, and a biotin appended complex
9
(Figure 12.9) shows preferential accumulation in the golgi apparatus
[21]. Rhenium complexes based on the [Re(CO)
3
(N^N)PyX] structure have now been reported that target a wide range of
cellular organelles including the nucleolus, golgi apparatus, ER, internal and plasma membranes, as well as mitochondria.
Complexes of this general structure are particularly amenable to the control of localisation and uptake as the basic core
[Re(CO)
3
(bipy)Py]
+
10
(Figure 12.10) appears to be poorly taken up by cells, with additional lipophilic substituents on the
pyridine required for good uptake (at least by passive diffusion), and the nature of these substituents determines localisation.
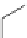
Small variations in the structure of the pyridine substituent can have a large effect on uptake and localisation. For example, a
X
+
Me
+
X
N
N
N
N
N
ReCl(CO)
5
N
Ag(I)
N
Re(CO)
3
Cl
Re(CO)
3
Re(CO)
3
N
N
N
N
sCHeMe 12.2
Synthesis of typical rhenium-based imaging probes.
Cl
N
OC
N
Re
OC
N
CO
8
FigUre 12.8
Mitochondrial targeting chloromethyl complex
8
.