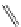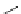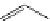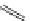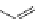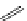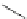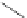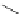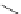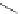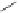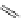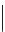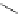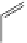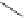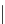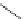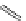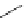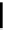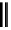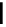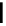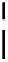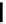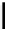Chemistry Reference
In-Depth Information
N
N
N
Ir
N
2
6
FigUre 12.6
Neutral, high pH form of imaging probe
6
.
N
O
CO
2
H
O
O
N
N
O
H
H
H
O
Ph
S
N
N
N
M
OC
CO
CO
7 M=Re/
99m
Tc
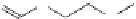
FigUre 12.7
Bisquinoline complex
7
.
There has been one report of the use of time-gated techniques with iridium cyclometallates in which a pH-responsive
iridium probe [Ir(ppy)
2
(pybz)]
6
(Figure 12.6) with a long lifetime was co-incubated in CHO cells with an organic nuclear
stain [11]. With no time-delay, the emission of the organic dye dominates, but with a 10 ns time gate before image acquisi-
tion, the cytoplasmic staining of the iridium complex is visible, because the short-lived organic fluorescence has decayed
away by this point. There are a small number of rhodium analogues of the iridium cyclometallates, which have been applied
in cell imaging and appear to show very similar patterns of uptake and localisation to the iridium cases [12].
12.2.2.3 Summary of Iridium/Rhodium Cell Imaging
Iridium cyclometallates have been established as promising
candidates for live cell imaging, with numerous examples showing good uptake, ideal photophysics with highly tunable
emission characteristics, and very long lifetimes. However, a propensity for widespread distribution throughout lipophilic
organelles may hinder the design of agents that target specific sites in the cell, which will be required for genuine biomedical
applications. Many examples also suffer from low aqueous solubility and significant cytotoxicity.
12.2.3
rhenium
fac
-tricarbonyl Heterocyclic Complexes as imaging Agents
12.2.3.1 Bisquinoline Complexes and Related Species
The first report of a rhenium
fac
-tricarbonyl heterocyclic com-
plex in cell imaging appeared in 2004 when Zubieta used the luminescence of a bisquinoline rhenium complex
7
(Figure 12.7)
conjugated to fMLF a peptide, which targets the formyl peptide receptor (FPR), to verify that conjugation of this unit to a
rhenium or technetium complex did not interfere with the receptor targeting [13]. Similar bisquinolines have since been
applied with other vectors, such as cobalamin B
12
[14], and the rhenium complexes of the dipicolyl amine analogues are often
used as cold analogues of their
99m
Tc complexes, which find use in SPECT imaging [15]. Many examples of similar complexes
based on the dipicolyl amine core with additional organic fluorophores have been used in cell imaging experiments to deter-
mine the cellular behaviour of Re/Tc complexes designed for radioimaging/therapy, but because the emission of these species
is not metal-based, they are better considered as examples of organic fluorophores [16]. Although the emissive bisquinoline
complexes were the first rhenium species reported as lumophores in cell imaging, typically their photophysical properties are
not as amenable to the technique as those of the bipyridyl and related complexes that have come to dominate the area.
12.2.3.2 Rhenium fac-Tricarbonyl Bipyridine Complexes
The most studied rhenium lumophores are based on a
fac
-
tricarbonyl Re
I
core with chelating N^N polypyridyl ligands. These species show photophysics dominated by emission from
a
3
MLCT state localised on the diimine ligand. The neutral complexes [ReX(bipy)(CO)
3
] (X = Cl/Br) typically show
maximum absorption at around 350 nm and emission at 500-600 nm. The luminescence lifetimes are around 100 s of ns, with
typical quantum yields around 0.1%. However, upon replacement of the halogen with a neutral (L = Py, PR
3
) ligand,