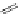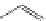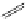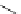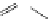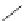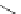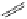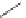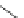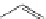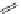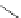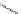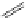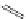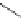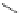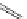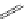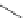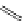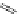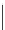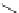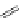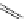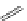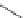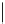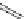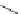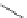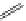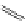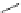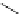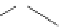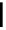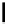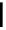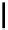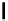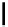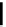Chemistry Reference
In-Depth Information
12.2.4
ruthenium and osmium Complexes in Cell imaging
Ruthenium complexes of the general structure [Ru(N^N)
3
]
2+
, where N^N represents a chelating polypyridine unit, (bipy,
phen) are the archetypal transition metal lumophores and have been widely studied in a variety of applications. Emission
emanates from a
3
MLCT excited state localised on the ligand framework, with all of the N^N ligands contributing to the
excited state (unlike the rhenium complexes in which only one ligand is involved). Typically, these complexes show excita-
tion maxima around 450 nm and emission maxima around 610 nm, with luminescence lifetimes of 0.6-6 µs and quantum
yields typically around 0.1, but up to 0.6%, making their photophysical properties ideal for cell imaging applications [24].
The sensitivity of the excited state to quenching by triplet dioxygen has led to the development of a range of ruthenium-
based oxygen sensors, with lifetime-based methods widely used to correlate lifetime and [O
2
] [25]. One major focus of the
studies of Ru
II
polypyridyls in biological systems has been the detection of dNA and other poly/oligo-nucleotides.
Complexes such as [Ru(bipy)
2
(dppz)]
2+
show only weak luminescence in aqueous solutions, but, upon intercalation of the
highly conjugated aromatic dppz ligand into dNA, enhancements of 10
4
-fold are observed [26]. While complexes with
these intercalating ligands have been shown to be excellent dNA sensors
in vitro
, uptake and localisation issues complicate
the issue
in vivo
[27].
12.2.4.1 Synthesis
Typically, ruthenium-based imaging agents are synthesised in a two-step sequence, with ruthenium
trichloride reacting with two equivalents of a bipyridine or similar ligand to give the neutral [RuCl
2
(N^N)
2
] species, which
are then converted by reaction with a different chelating polypyridine to the cationic [Ru(N^N)
2
(N'^N')]
2+
agents
(Scheme 12.3). Usually, the unique N'^N' ligand is chosen for the specific localisation or sensing properties desired, and the
two N^N ligands are chosen to tune the overall photophysical properties.
12.2.4.2 Development of Ruthenium Imaging Agents
The uptake of simple ruthenium complexes is low, and in several
cases it has been found that although they are taken up by active transport, the luminescence is only evident in endosomes
from which it appears that the complexes do not escape. The first reports [28] of the application of ruthenium in cell imaging
concentrated on mapping oxygen concentration in cells using [Ru(bipy)
3
]
2+
in FLIM. Because this technique relies only on
lifetime, and not intensity, the relative concentrations of the complex in different cellular compartments are irrelevant.
Subsequent studies [29] highlighted problematic uptake, with complexes such as [Ru(phen)
3
]
2+
being unable to cross healthy,
intact membranes, leading to encapsulation in endosomes. In order to overcome problems, a range of complexes bearing
substituents known to assist in uptake such as polyarginines have been prepared [30].
Barton has conducted a systematic study of complexes bearing dppz and related ligands as cellular dNA probes. In many
cases the problems of uptake have been overcome by appending more lipophilic units to assist membrane transport, and
other units such as oligoarginines and fluorescein have also been introduced [31]. A series of complexes of the general for-
mula [Ru(dppz)(N^N)
2
]
2+
,
15
allowed a correlation between the lipophilicity of the N^N unit and the degree of uptake to be
established [32]; this has been shown to be by a passive mechanism [33]. Highly lipophilic analogues of simple dppz
complexes based around alkyl-ether substituted dppz ligands (Figure 12.12) developed by Svensson all showed good uptake.
2+
N
N
N
Cl
N
N
dppz
RuCl
3
Ru
Ru
Cl
N
N
N
N
N
2
2
sCHeMe 12.3
Synthesis of ruthenium imaging agents.
N
N
O
N
R
R
Ru
N
O
N
N
2
FigUre 12.12
Lipophilic Ru dppz analogues
15a
(R = Et) b (R = Bu) c (R = hex).