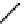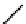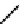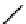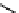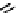Chemistry Reference
In-Depth Information
electron-deficient conjugated region in the excited molecule. According to the Franck-Condon principle, these two conjugated
regions can remain co-planar in the locally excited state. Solvent relaxation then takes place with a concomitant rotation
around the sigma bond connecting the electron-rich and electron-poor parts of the excited fluorophore until they are orthog-
onal to each other. This results in a twisted internal charge transfer (TICT) state with complete charge separation (Figure 11.28).
In addition to the fluorescence band due to the emission from the locally excited state ('normal' band), an additional emission
band corresponding to emission from the TICT state can be observed at longer wavelengths ('anomalous' band) [128].
11.4.5
excited-state proton transfer
In comparison with its ground state, a photo-excited molecule may possess different acid-base properties. The redistribution
of the electronic density upon photo-excitation is one of the reasons for such a phenomenon. If an excited species is a strong
acid or base compared with its ground state, photo-induced proton transfer may take place. The acidic character of a proton
donor (e.g., the hydroxyl substituent of an aromatic ring) can be enhanced upon excitation so that its pK* becomes much
lower than that of its group state (Figure 11.29a). In the same way, the pK* of an acceptor group (e.g., a heterocyclic nitrogen
atom or carboxylate) in the excited state can be much higher than that of its ground state (Figure 11.29b) [129].
The excited state proton transfer (ESIPT) process can be easily recognised in steady-state spectra: The absorbance is gen-
erally similar to that of the parent chromophore, but the fluorescence is significantly different. ESIPT dyes generally show
large Stokes' shifts and are ideal candidates for being used as fluorescence labels to avoid interference from other fluorescent
materials present in the specimen. An advantage of the large Stokes' shift is the almost complete lack of spectral overlapping
between absorption and emission, which makes ESIPT dyes promising for their use in fluorescent chemosensing. Very
recently, a sensor based on a
bis
(benzoxazole) derivative (Figure 11.30) in which metal binding enables the ESIPT
Full charge
separation
(TICT state)
+
-
Partial charge
separation
(locally excited state)
δ
+
δ
-
ICT
A
D
Ground state
fIgure 11.28
Charge separation in the ICT state and the TICT state.
(a)
(b)
BH*
B*
-
+ H
+
B* (or B*
-
) + H
+
B*+ (or B*)
pKa / pKa*
pK / pK*
OH
NH
2
10.6 / 3.6
7.1 / 12.2
CO
-
OH
9.3 / 2.8
3.7 / 6.9
OH
9.12 / 1.66
5.5 / 10.6
-
O
3
S
N
fIgure 11.29
Photo-induced changes in the acid-base properties of chromophores: (a) photo-induced enhancement in acidity result-
ing in excited state deprotonation, and (b) photo-induced enhancement in basicity resulting in excited state protonation.