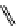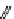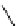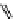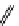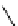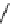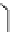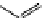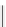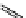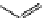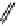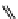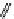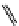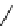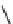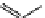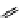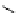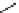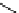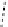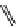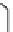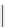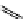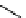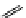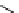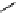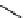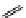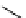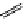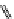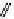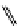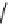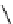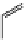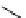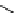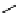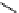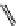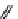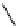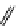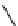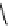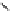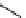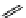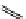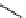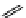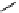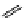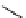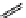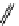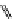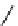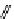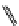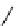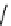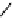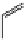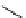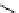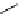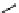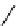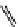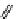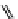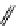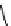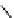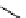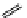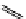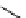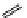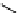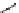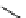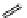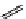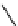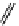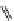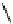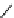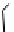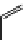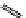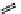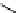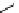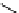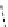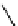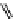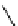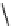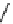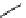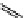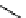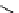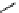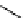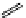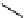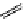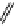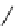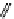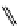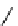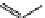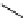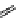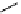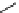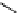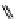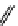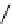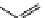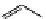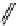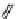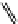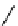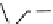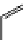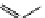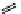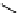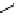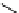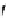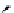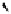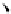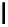Chemistry Reference
In-Depth Information
N
N
N
N
N
N
H
Zn
N
N
N
N
N
N
N
N
N
N
N
N
Zn
2+
O
O
O
-
O
O
O
O
O
O
H
+
Cl
Cl
Cl
Cl
Cl
Cl
CO
-
CO
-
CO
-
Weakly
uorescent
Weakly
uorescent
Weakly
uorescent
Zn
2+
Zn
2+
H
+
H
+
N
N
N
N
N
N
Zn
H
Zn
H
Zn
H
N
N
N
N
N
N
N
N
N
N
N
N
O
O
O
O
O
O
O
O
O
Cl
Cl
Cl
Cl
Cl
Cl
CO
-
CO
-
CO
-
Strongly
uorescent
Strongly
uorescent
Strongly
uorescent
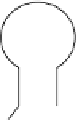
fIgure 11.25
The PET chemosensor Zinpyr-1 for Zn
2+
[56].
+Exo-or endonuclease
Fluorophore “turns on”
Fluorophore “turns off”
Fluorophore “turns on”
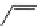
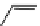
fIgure 11.26
An application of the PET mechanism for smart biosensors. A fluorophore is attached to 5′ end of the oligonucleotide
and quenched by the guanine residues of the complementary stem. upon hybridisation to the target sequence, or exo-/endo-nucleolytic
digestion, fluorescence of the fluorophore is restored.
M*
+M
(MM)*
(Excimer)
D*
+ A
+D
(DA)*
(Exciplex)
(Exciplex)
A*
(AD)*
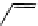
fIgure 11.27
Excimer and exciplex formation.
redistribution of electron density and the creation of a substantial dipole. Consequently, the excited state, upon excitation
(known as the Frank-Condon state or the locally excited state) is not in equilibrium with the surrounding polar solvent
molecules. For a fluorophore with two co-planar π-conjugated moieties joined together by a σ-bond, photo-excitation
and subsequent intramolecular transfer of an electron from one moiety to the other generates an electron-rich and an