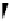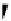Chemistry Reference
In-Depth Information
(a)
LUMO
LUMO
LUMO
LUMO
HOMO
HOMO
HOMO
HOMO
PET
Excited
uorophore
(A*)
Electron-rich
quencher
(D)
Reduced
uorophore
(A
• -
)
Oxidised
quencher
(D
• +
)
+
+
(b)
LUMO
LUMO
LUMO
LUMO
HOMO
HOMO
HOMO
HOMO
PET
Excited
uorophore
(D*)
Electron-poor
quencher
(A)
Oxidised
uorophore
(D
• +
)
Reduced
quencher
(A
• -
)
+
+
fIgure 11.24
The PET mechanism: (a) reductive and (b) oxidative electron transfer.
sensitivity for fluorescence-based chemosensing of minute amount of target analytes. A typical PET fluorescent probe con-
sists of a fluorophore and a quencher moiety connected together through a spacer. The intramolecular PET process keeps the
probe in the 'off' status at rest. In the presence the targeted analyte, its specific interaction with the intramolecular quencher
moiety turns off the PET process and 'turns on' the fluorophore to produce a strong fluorescent signal. Figure 11.25 shows
a PET chemosensor Zinpyr-1 for Zn
2+
ions that contains a 2′,7′-dichlorofluorescein fluorophore and two dipicolylamine
moieties for Zn
2+
binding [125]. The lone pairs of electrons on the tertiary amines of dipicolylamine act as the PET quencher.
Fluorescence of the chemosensor is 'turned on' upon binding with Zn
2+
or H
+
by the dipicolylamines.
The PET process is also useful in biosensing, where specific fluorescence quenching of fluorophores by selected
quenchers such as naturally occurring DNA nucleotides (e.g., guanine) and amino acids (e.g., tryptophan) is adopted to
probe the presence of target molecules (Figure 11.26). With careful design of these conformationally flexible biosensors,
efficient PET-probes with single molecule detection sensitivity can be produced [126].
11.4.3
excimer/exciplex formation
Excimers are excited state dimers, which are formed by the collision between an excited molecule and a ground state mole-
cule of the same species. Exciplex are excited-state complexes that are formed by the collision of an excited molecule with
a ground state molecule of another species, forming excited-donor/acceptor or excited-acceptor/donor complexes
(Figure 11.27). These excimer/exciplex formations are diffusion-controlled processes. In polar media, de-excitation of exci-
plexes can lead to fluorescence emission of ion pairs and subsequently 'free' solvated ions. Therefore, exciplexes can be
considered as intermediate species in electron transfer from a donor to an acceptor in some cases. The lifetime of excimers/
exciplexes is very short, on the order of nanoseconds, and their corresponding fluorescence are usually broad and red-shifted.
This mechanism normally occurs at high concentration due to the increased probability of forming the excimer [127].
11.4.4
Internal charge transfer and twisted Internal charge transfer
Excitation of a fluorophore promotes an electron from one molecular orbital to another one of higher energy. If the initial
and the final molecular orbitals are located on different parts of the fluorophore, the electronic transition will be accom-
plished by an almost instantaneous change in the molecular dipole moment. In such a manner, excitation gives rise to a