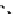Chemistry Reference
In-Depth Information
11.4 MechanIsMs of photophysIcal processes and theIr applIcatIons
In Molecular IMagIng and cheMosensIng
Photophysical properties of fluorophores are strongly influenced by a variety of factors such as the viscosity, polarity, and
rigidity of the media and the bio/macromolecules on which they are tagged; the presence of quenchers that interact with the
fluorophores at either their ground or excited states; and their proximity to energy donors. Detailed information about the
chemical nature of the surroundings of a fluorescent probe can be obtained by thorough interpretation of its luminescent
behaviour. Fluorescent probes with specially incorporated features, such as analyte-specific receptors, energy/electron
donor-acceptor pairs, can be utilised to selectively reveal certain important characteristics of the system under investigation.
Therefore, fluorescent techniques making use of various kinds of fluoro-tags, fluorescent probes, and chemosensors have
been becoming increasingly eminent to life science and biomedical studies ever since the advent of fluorescent microscopy
and subsequently laser scanning confocal microscopy to the research communities. The following is a brief introduction
to the fundamental principles of various fluorescent techniques that are commonly applied in bioimaging and
in vitro
and
in vivo
bio- and chemosensing.
11.4.1
fluorescence resonance energy transfer
The extra energy possessed by an excited fluorophore in a more energetic excited state can be transferred to and, hence,
excites another fluorophore in a lower energy excited state. There are generally two possible pathways for such energy
transfer—the
radiative
and
non-radiative
decay. In radiative energy transfer, an emitted photon from a donor molecule is
reabsorbed by an acceptor. In this case, energy is transferred directly through long-range (1-10 nm) dipole interactions
between a donor and an acceptor. This can occur if the emission spectrum of the donor overlaps with the absorption spectrum
of the acceptor, given that several vibronic transitions in the donor have practically the same energy as the corresponding
transitions in the acceptor (Figure 11.23) [123]. Because fluorescence resonance energy transfer is highly sensitive to the
distance between the donor fluorophore and the acceptor fluorophore, it can be used as a spectroscopic ruler to measure
intermolecular interactions in the range of 10-100Å.
11.4.2
photo-induced electron transfer (pet)
Photo-induced electron transfer (PET) is often responsible for the quenching of fluorescence from excited fluorophores.
This can be mediated by either oxidants or reductants. As illustrated in Figure 11.24, reductive electron transfer quenching
involves the transfer of an electron from the HOMO of an electron-rich quencher (usually a lone pair of electrons on a hetero
atom) to the “hole” left in the HOMO of an excited fluorophore. Similarly, the excited electron of an excited fluorophore can
be transferred to the LuMO of an electron-deficient quencher, resulting in an oxidative electron transfer quenching. Like
FrET, PET is another photophysical process that leads to the variation of fluorescence efficiency by distance-dependent
fluorescence quenching between a fluorophore and a quenching moiety [124]. This PET mechanism offers extraordinary
Vibrational relaxation
Fluorescence
resonance
energy transfer
S
1
S
1
S
o
S
o
Donor
Acceptor
fIgure 11.23
Illustration of fluorescence resonance energy transfer.