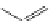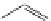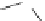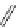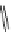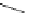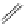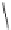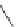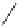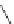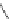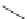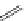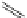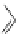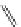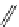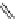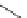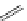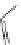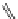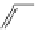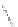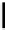Chemistry Reference
In-Depth Information
HO
HO
N
O
N
O
O
N
O
N
O
-
Zn
2+
OH
Zn
2+
N
N
N
N
N
N
fIgure 11.30
A Zn
2+
sensor based on excited-state intramolecular proton transfer (ESIPT) [130].
bis
(benzoxazole) derivative to give a new emission band in the NIr region with a large Stokes' shift (ca. 230 nm) has been
reported [130]. The fluorescence of the free ligand is quite weak (
λ
em
= 543 nm;
Φ
fl
= 0.0067), possibly due to the presence of
its deprotonated form, which exists in a small amount in equilibrium with its neutral form in the polar media. upon addition
of Zn
2+
, the intensity of the emission signal at 543 nm increased gradually, and a new emission band appeared in the NIr
region at 712 nm. Other metal ions (Cd
2+
and Hg
2+
) give rise to different changes in the fluorescent behaviour as they per-
turbed the enol and keto emission of
bis
(benzoxazole) derivative to different degrees.
11.5
two/MultI-photon Induced eMIssIon and
In VITro
/
In VIVo
IMagIng
11.5.1
principles of two-photon Induced emission and Imaging
In the previous sections, we have looked at a variety of organic-based colorimetric and fluorescent staining agents, probes
and chemosensors and their applications in bioimaging. Most of these bio-bioimaging/biolabelling agents are single-photon
fluorophores. Their operational spectral range is generally in the uV and visible region, which has low tissue penetration
ability. Also, their relatively short fluorescent lifetimes sometimes makes it difficult for researchers to distinguish their sig-
nals from background autofluorescence in cells and tissues. This situation was changed in 1990 by the pioneering work of
Webb and his co-workers on two-photon laser scanning fluorescence microscopy that made use of the lower energy, but
much greater tissue-penetrating near-infrared (NIr) radiation as the excitation source [131]. Advantageous features of multi-
photon bioimaging include a reduction in photobleaching and photodamage to the imaging probes and cellular structures,
the capability to penetrate thick tissues, and the ability to bring about precise three-dimensional localised photosensitisation,
photolysis, ablation, and cutting at the subcellular level. Even though the two-photon absorption in some organic materials
can be as high as 100,000 GM (GM = 10
-50
cm
4
s photon
-1
) (Figure 11.31), there are very few materials that can be used for
in vitro/in vivo
imaging. It is only in the last decade that a few two-photon excitable organic dyes become commercially
available.
The elementary processes of linear and two-photon absorptions are both induced by a single laser beam. The schematic
diagram for these processes in the regime of quantum theory is shown in Figure 11.32. An intermediate state (schematically
represented by a dashed line) is introduced between the two real eigenstates (i.e., the ground and excited states) of a mole-
cule. The occurrence of two-photon absorption, inducing the molecular transition between its two real states, can be visu-
alised as a 'two-step' event: (i) In the first step, one photon is absorbed while the electron of molecule leaves its initial state
E
g
and be promoted to an intermediate state; (ii) in the second step, another photon is absorbed for the same electron to
complete its transition from the intermediate state to the final real state
E
f
. The key connection between these two steps is
the intermediate state in which the molecular status is not certain in the sense that the molecule may stay in all of its pos-
sible eigenstates (except
E
g
and
E
f
) with a certain probability of distribution. When a two-photon fluorophore absorbs light
energy, it is often excited to a higher vibrational energy level in the first excited state, S(1), before rapidly relaxing to the
lowest vibrational energy level. This event, depicted as a 'stair-step' transition from the upper to lower vibrational energy
levels in S(1), is termed vibrational relaxation or internal conversion and takes place within a picosecond or less [134, 135].
Typically, fluorescence lifetimes are of more or less four orders of magnitude shorter than vibrational relaxation, giving the
molecules sufficient time to achieve a thermally equilibrated lowest-energy excited state prior to fluorescence emission.
The two-photon absorption cross-section is one of the parameters for the evaluation of the efficiency of a two-photon pro-
cess. Z-scan and comparison with relevant fluorescence standards are the two commonly adopted measurements for the
evaluation of it [136].
Since 1990, many classes of materials have been found capable of participating in direct two- or multi-photon absorption
processes and become potential candidates for
in vitro
and
in vivo
bioimaging [137, 138]. Here, we will limit our discussion