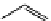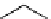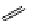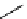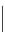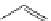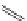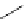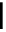Chemistry Reference
In-Depth Information
O
Xanthene
Important xanthene-based uorescent
biolabelling agents
λ
abs
(nm)
λ
em
(nm)
Remarks
Rhodamine B (aminoxanthene dye)
548
627
Acidic aqueous EtOH
Fluorescein isothiocyanate (FITC)
494
519
pH 9.0
Tetramethylrhodamine isothiocyanate (TRITC)
537-555
564-580
pH 8.0
Oregon green 514 ester
506
526
pH 9.0
Texas red
587
602
Chloroform
fIgure 11.11
Structure of xanthene.
N
Acridine
Acridine-based dyes
λ
abs
(nm)
λ
em
(nm)
Remarks
502 nm when bound with DNA,
650 nm when bound with RNA
Acridine orange (AO)
492
535
Acriflavine,
452
510
/
Quinacrine
445
500
/
fIgure 11.12
The acridine dyes.
The isothiocyanate, succinimidyl ester, and sulfonyl chloride moieties of these 'reactive dyes' enable them to form covalent
linkages with the free, and exposed, amino moieties in peptides, proteins, and antibodies for their fluoro-tagging.
11.3.4
acridine- and phenanthridine-based luminophores
Both acridines and phenanthridines contain a central pyridine ring fused with two benzo rings, one on each side (Figures 11.12
and 11.13). They are small cationic and planar dyes that are able to interact with DNA and rNA via intercalation and cou-
lombic attraction [69, 70]. This makes acridine and phenanthridine luminophores useful nucleic acid selective stains for cell
cycle determination [71-74]. Important acridine dyes include Acridine Orange, Acriflavine, and Quinacrine. Acridine Orange
(AO) was first reported as a fluorescent microscopy stain in 1940 [75-77]. It can enter both live and dead cells and has unique
properties in differentiating between DNA and rNA as it gives green fluorescence (λ
max
at 502 nm) when bound to DNA, and
orange fluorescence (λ
max
at 650 nm) when bound to rNA. Acriflavin is used as a general oversight stain in fluorescent
microscopy in entomological specimens [78]. It can also be used in the
in vivo
imaging of rat brain by laser scanning con-
focal microscopy [79]. Quinacrine is a decent stain for lysosome and nucleic acid [80, 81]. It can also stain platelets as they
store the dye in dense granules. This is useful for the assessment of platelet adhesion and aggregation [82-84].
Perhaps the most important phenanthridine dye for bioimaging is Ethidium Bromide (EB). It is useful for the detection of
nucleic acids and the staining of cell nuclei because it can intercalate into DNA helices, similar to that of acridine dyes, to give
strong orange colour fluorescence. However, it only interacts weakly with rNA to give a weak red fluorescence. It is often used
in combination with AO to reveal the viability status of cells [85, 86]. Both live and dead cells can take up AO, while EB can
only enter dead cells and their nuclei via their proliferated plasma and nuclear membranes. Therefore, under the co-staining of
AO and EB with blue light excitation, viable cells will show bright green nuclei and red cytoplasm, while dead cells will show
bright orange nuclei and cytoplasm (as fluorescence of EB overwhelms that of AO). Any remaining rNA will appear dark red.
Nuclei of both live and dead cells have well-defined euchromatin and heterochromatin and will appear as distinct structures
under the co-staining of AO and EB. However, apoptotic nuclei possessing highly condensed chromatin will appear as uniformly
stained bodies. Also, in advanced apoptosis, cell nuclei will only be weakly stained, because the DNA in the nuclei is gone.
Another phenanthridine dye (Figure 11.13) that has similar properties to EB is Propidium Iodide (PI). It is often used in
combination with a benzimidazole dye, Hoechst 33342 (Hoe 33342), to report the cell viability status [87, 88]. Nuclei of
the viable cells will be stained blue (by Hoe 33342), while those of the dead cells will appear bright pink (a blend of fluo-
rescence from Hoe and PI). Cytoplasm of the viable cells is free from staining (all Hoe enters cell nuclei and PI cannot
penetrate the plasma membrane), while that of the dead cells will be stained bright pink (same as their nuclei).