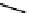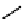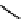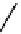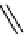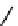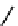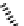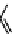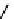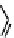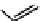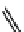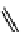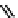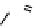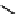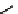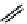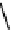Chemistry Reference
In-Depth Information
R
2
1
R
2
R
2
N
N
N
+
N
e
e
N
N
2
5
R
5
R
5
H
R
5
N
N
N
N
N
N
3
R
3
R
3
4
R
3
Tetrazolium
Tetrazolinyl
Formazan
+
Reductases
N
N
N
N
-
Cl
H
N
N
S
N N
N
S
λ
abs
=
378 nm
λ
abs
=
560 nm
N
MTT
MTT formazan
fIgure 11.10
The tetrazolium-formazan transformation and the methylthiazolyldiphenyl tetrazolium assessment of metabolic
viability of cells.
in the injured central nervous system [55, 56]. Similarly, the high affinity of Evans Blue for plasma albumin has made it a
useful probe for the measurement of the blood volume of laboratory animals and humans [57-59]. It can also act as a
fluorescent tag (fluoro-tag) to plasma albumin in the assessment of the integrity of the blood-brain barrier. If the blood-brain
barrier has been compromised, the normally excluded serum albumin can penetrate into the brain. Such an event can be
revealed by the albumin bound Evans Blue tag that fluoresces at 680 nm upon excitation at 470 nm [60].
11.3.2.1 Tetrazolium Compounds
Tetrazolium compounds, which possess an electron-deficient five-membered hetero-
cyclic ring, can easily be reduced by reductase enzymes to the highly coloured and less water-soluble formazans (Figure 11.10)
[61, 62]. Therefore, they can become useful colorimetric probes for general metabolic activities and the viability of cells.
The most famous tetrazolium compound for this application is methylthiazolyldiphenyl tetrazolium (MTT) (Figure 11.10).
MTT assay has been extensively adopted in the field of cell biology for the assessment of cell viability [63, 64]. The
absorption maxima of MTT shifts from 378 nm (yellow) to 560 nm (magenta) upon reduction to MTT formazan.
With the rapid progress of fluorescent techniques in histochemistry, cell, and molecular biology since the early 20th
century, a number of organic chromophores that were established centuries ago have found new utilities under the newly
developed fluorescent microscopy and the subsequent confocal laser scanning microscopy. As highlighted in the previous
section, these fluorescent organic dyes form the basic building blocks for the fluoro-tags for antibodies, proteins, and other
biomolecules, as well as signalling units in chemosensors to report analyte-binding events. Some of their spectrofluoro-
metric and photophysical properties have also been found to be extremely useful for the design of advanced probes to reveal
complex histo- and cytochemical interactions. Here, we will focus our discussion on a number of important families of fluo-
rophores, such as xanthenes, acridines and phenanthridines, and polymethines, as well as some miscellaneous fluoro-tags,
such as NBD chloride, dansyl chloride, BODIPY, and its derivatives [65, 66].
11.3.3
Xanthene-based luminophores
As introduced in the previous section, xanthene dyes are important fluoro-tags because of their outstanding photophysical
properties and biocompatibility (Figure 11.11). Both anionic hydroxyxanthenes, for example, Fluorescein, and cationic ami-
noxanthenes, for example, rhodamine B, are commonly used luminophores for the labelling of biomolecules and as signal-
transducers in the chemosensing of specific analytes. For example, the famous Calcium-Green
TM
-1, for real-time
in vitro
and
in vivo
calcium flux imaging, makes use of a fluorescein moiety as the signal transducers to report the binding of calcium
ions [67, 68]. Given their relatively low energy fluorescence (emission maxima >500 nm), they are also widely used as energy
acceptors in Fluorescent resonance Energy Transfer (FrET) processes. In fact, a number of xanthene derivatives that carry
reactive substituents are routinely being used as biolabelling agents in biomedical and cell biology research. These include
Fluorescein isothiocyanate (FITC), tetramethylrhodamine isothiocyanate (TrITC), Oregon Green 514 Ester, and Texas red.