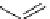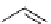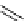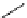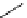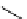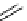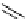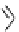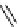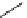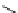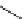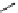Chemistry Reference
In-Depth Information
N
Phenanthridine
Phenanthridine-based dyes
λ
abs
(nm)
λ
em
(nm)
Remarks
Ethidium bromide (EB)
480
605
620 nm when bound with DNA
Propidium iodide (PI)
493
636
617 nm when bound with DNA
fIgure 11.13
The phenanthridine dyes.
R
3Cl
-
H
+
N
NH
+
N
N
+
H
2
N
N
H
NH
2
NH
NH
2
2Cl
-
H
DAPI (λ
abs
= 344 nm; λ
em
= 450 nm,
461 nm when bound to DNA)
Hoechst 33342
(λ
abs
= 350 nm; λ
em
= 461 nm,
461 nm when bound to DNA)
Hoechst 33258
(λ
abs
= 352 nm; λ
em
= 461 nm,
461 nm when bound to DNA)
R=OEt
R=OH
fIgure 11.14
The indolenine- and benzimidazole-based DNA probes DAPI, Hoechst 33342, and Hoechst 33258.
11.3.5
polymethine-based luminophores
The basic structure of polymethine luminophores consists of an electron donor and an electron acceptor moiety separated by
a conjugated carbon chain or spacer group. Polymethines can be classified into a number of sub-families, such as indolenines
and benzimidazoles, cyanines, styryls, and coumarins. Two sets of famous indolenine- and benzimidazole-based polyme-
thine fluorescent probes for bioimaging are 4′,6-diamino-2-phenylindole dichloride (DAPI) and Hoechst (Hoe) stains
(Figure 11.14). Both are live-cell compatible DNA probes that can be used to stain cell nuclei. DAPI is an indolenine dye
that gives bright blue colour fluorescence when bound to DNA [89]. It has a strong affinity for the A-T rich regions of DNA
strands. However, it can also bind to rNA to give weak fluorescence at around 500 nm.
The Hoechst stains are benzimidazole dyes that also possess high affinity for the A-T rich regions of DNA. They are
relatively less toxic and more cell permeable than DAPI. Fluorescence from Hoechst stains can be quenched by bromode-
oxyuridine (Brdu). The latter can be used by cells as a substituent for thymidine in DNA synthesis. The Hoechst-Brdu pair
can, therefore, be used to detect dividing cells. During the S-Phase of the cell cycle before mitosis, DNA in the cell nuclei is
replicated, and chromosomes are duplicated. If cells have been given Brdu instead of thymidine, they will incorporate the
Brdu into their newly synthesised DNA. These Brdus quench the fluorescence of Hoechst dyes, which results in the absence
of the blue fluorescence from cell nuclei that are about to undergo mitosis [90, 91]. Another use of Hoechst stains is for
the assessment of cell viability, in combination with the live cell impermeable Propidium Iodide (PI) dye. This technique has
already been outlined in the previous section.
Cyanine stains are polymethine dyes that possess nitrogen-based electron donors and acceptors. Famous examples of
these fluorescent probes in bioimaging applications include Merocyanine 540, CellTracker
TM
CM-DiI, and Cy3 & Cy5
fluoro-tagging agents. Merocyanine 540 (Figure 11.15) is the first electrochromic dye used for the imaging of membrane
potential. It is a slow-response membrane-potential probe that binds to the surface of polarised membranes in a perpendic-
ular orientation, but dimerises into non-fluorescent dimers upon membrane depolarisation [92-94].
The CellTracker
TM
CM-DiI (Figure 11.16) is a symmetrical cyanine i.e., both the electron donor and acceptor end-groups on the
conjugated carbon chain are identical, with excellent cell retention and minimal cytotoxicity. This cyanine membrane stain can be
used for long-term labelling and tracking of live cells, intracellular membranes, liposomes, viruses, and lipoproteins [95-97]. The
stain can be retained on the cell membranes throughout fixation and permeabilisation steps. It has two lipophilic C
18
-carbon chains
and a mildly thiol-reactive chloromethyl moiety for the labelling of membranes and thiol-containing peptides and proteins.