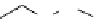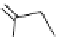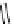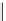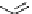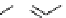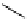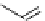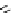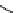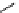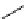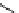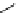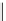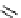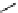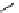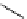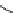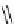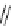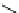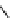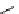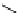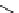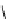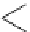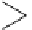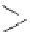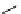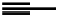Chemistry Reference
In-Depth Information
(a)
Biomolecule
Biomolecule
R+H
2
N
X
Fluorescent
dye
Fluorescent
dye
S
S
C
H
N
N
l
N
R=
X=
NN
NN
R
ʹ
R
ʹ
O
O
O
N
H
O
O
(b)
R+HS
Biomolecule
X
Biomolecule
Fluorescent
dye
Fluorescent
dye
O
O
S
N
N
R=
X=
O
O
O
O
I
S
H
H
(c)
N
N
N
Biomolecule
Biomolecule
N
3
+
Fluorescent
dye
Fluorescent
dye
(d)
Biomolecule
Biomolecule
+
Fluorescent
dye
Streptavidin
Fluorescent
dye
Streptavidin
= Biotin
fIgure 11.2
Fluorescence labelling via (a) amino groups, (b) sulfhydryl groups, (c) click chemistry, and (d) streptavidin-biotin bridges.
chemistry—the [3 + 2] cycloaddition between azides and alkynes. Alternatively, both the fluorescent dye and the biomolecule
can be labelled with biotin and then coupled together via the formation of the streptavidin-biotin bridges.
Despite the rapid growth in the field of bioconjugation, tagging of biomolecules with specific fluorescent probes is still
a very challenging task, and hence, a lot of the bioimaging studies nowadays are carried out with 'non-covalently associ-
ating' probes. Without prior anchorage to any biomolecules, the histological and cellular localisation profiles of these non-
covalently associated probes are directed by their chemical nature, as well as the establishment of specific interactions within
the system under investigation. Hydrophilicity/hydrophobicity and acid-base characteristics of these fluorescent probes are
two important properties governing their
in vitro
and
in vivo
behaviour. For example, a series of commercially available