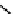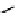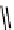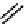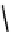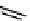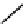Chemistry Reference
In-Depth Information
background. Expression of fluorescent proteins in genetically modified cells is another frequently adopted approach for the
visualisation of specific protein transcription processes in cells [10-14]. Nevertheless, the majority of biomolecules and intra-
cellular components do not possess any special spectroscopic or luminescent properties for their easy observation and study.
Such an approach cannot reveal any post-transcriptional information. Thus, the use of exogenous chromophores and lumino-
phores for direct staining of intracellular components, in live and proliferated cells, and fluoro-tagging/labelling and tracing
of biomolecules
in vitro
, is still the most common microscopic imaging practice in biomedical research and cell biology.
From the dawn of optical microscopy in the 17th century to the present, state-of-the-art near-infrared and multi-photon
confocal laser scanning microscopy, staining/labelling agents, and probes ranging from extracts of natural plants and insects
to simple inorganic salts, synthetic organic dyes, coordination and organometallic complexes, and nanoparticles and nano-
composite materials have been developed for many purposes. The scope of this chapter is to introduce readers to a series of
specially designed organic-based dyes and probes that are useful in bioimaging and
in vitro
/
in vivo
chemosensing to reveal
the locales and conditions of specific subcellular structures, the activity of selected cytochemical processes, and the
in vitro
syntheses, trafficking, interactions, and degradations of specific biomolecules.
11.2
desIgnIng Molecular probes for bIo-IMagIng
Before the turn of the 20th century, colorimetric staining agents were the only tools available for cell biologists. The inven-
tion of fluorescent microscopy in 1904, its subsequent popularity in the life science community in the late 1970s and early
1980s, and the eventual emergence of the laser scanning confocal microscopy in 1986 revolutionised research in cell biology.
The discoveries enabled the development of a suite of novel techniques to divulge cellular architectures and intracellular
parameters with high optical resolution that had never been revealed before. Designated subcellular features can now be
made to glow at preselected wavelengths with the corresponding fluoro-tagged antibodies. Intra- and intercellular transpor-
tation of metal ions and biomolecules can now be followed in real time with the use of specific luminescent chemosensors.
Histochemical processes taking place deep within thick tissues can now be observed and analysed by optical sectioning with
laser scanning confocal microscopy and deep tissue-penetrating near-infrared and multi-photon imaging techniques.
Biomolecules that bear natural fluorophores are ideal subjects for bioimaging because they can be directly observed at
the appropriate excitation and observation wavelengths, with minimal perturbations to the biological system under investi-
gation. Common natural fluorophores present in proteins are aromatic amino acids, including tryptophan, tyrosine, and
phenylalanine (Figure 11.1). Among these three, tryptophan is the most highly fluorescent. However, its quantum yield in
different proteins can vary from < 0.01 to 0.35 with lifetimes from < 0.1 to 7 ns. Such luminescent efficiency is much poorer
than those of most other conventional fluorescent dyes. Another serious limitation of native fluorescence detection of aro-
matic amino acids is their low photostability under single- and two-photon excitation. All these have rendered native fluoro-
phores not suitable for highly sensitive applications, such as single-molecule fluorescence detection.
With these limitations of natural fluorophores, the use of exogenous synthetic organic fluorophores (fluorescence dyes)
remains the mostly adopted approach in bioimaging, biomedical, and bioanalytical studies. There are two general tactics in
the application of synthetic organic fluorophores: (a) the
covalent association approach
—the fluorophores are covalently
tagged onto the targeted biomolecules so that their transportation and transformation can be traced, and (b) the
non-covalent
association approach
—the fluorophores are not covalently bound to any biomolecule prior to
in vitro
/
in vivo
applications
and are free to engage in any interaction with various biochemical species and bio-transformation processes.
As shown in Figure 11.2, tagging of a fluorescence dye covalently to an analyte biomolecule can be achieved using
(a) amine-reactive functional moieties such as isothiocyanates, chlorotriazinyl derivatives, and hydroxysuccinimido
active esters; (b) sulfhydryl-reactive moieties such as iodoacetamido and maleimido functional groups; and (c) via click
Phenylalanine
H
H
3
+
NCCO
2
-
R
R=
Tyrosine
OH
Tryptophan
H
fIgure 11.1
Structures of naturally fluorescent aromatic amino acids.