Chemistry Reference
In-Depth Information
thus allowing the separate visualisation of nanomolar concentrations of both agents in the same image voxels. Subsequently,
two lipoCEST agents were injected under the skin of a mouse in different body regions and were successfully detected inde-
pendently upon irradiation at the respective absorption frequencies of their exchangeable proton pools [75].
More recently, preliminary results on
in vivo
targeting of α
v
β
3
integrin receptors (which are known to be overexpressed
during angiogenesis in many tumour vessels) have been reported by Flament et al. [76] In this study, an RGD-functionalised
lipoCEST agent was prepared and injected intravenously in a mouse model bearing a u87 glioma brain tumour. An enhance-
ment in CEST contrast in the tumour and in other brain regions was observed. This was attributed to nonspecific binding
and/or distribution of the RGD-lipoCEST. Evidently, these studies highlight the feasibility of the
in vivo
detection of targeted
lipoCEST agents, even though the issue of specificity needs to be addressed further.
Opina et al. have tackled the task of endowing lipoCEST agents with pH responsiveness by encapsulating lanthanide
complexes of the DOTA-tetraglycinate ligand (endowed with four magnetically equivalent amide protons that exchange with
protons of bulk water) in liposomes [77]. The base-catalysed amide proton exchange of the respective complexes followed
the order Yb > Tb > Dy. Even though the Dy(III) complex showed the largest hyperfine shift, the combination of favourable
chemical shift and amide proton CEST linewidth in the Tm(III) complex was deemed more amenable for future
in vivo
applications where tissue magnetisation effects can interfere.
TmDOTA-(gly)
4
-
at various concentrations was encapsulated in the core interior of liposomes to yield lipoCEST particles
for molecular imaging. The resulting nanoparticles showed less than 1% leakage of the agent from the interior over a range
of temperatures and pH values. A plot of the magnitude of the CEST effect from the amide proton exchange as a function of
pH differed for the free versus encapsulated agents over the acidic pH regions, consistent with a lower proton permeability
across the liposomal bilayer for the encapsulated agent. Nevertheless, the resulting lipoCEST nanoparticles amplified the
CEST sensitivity by a factor of approximately 10
4
compared to the free, unencapsulated agent. Such pH sensitive nano-
probes could prove useful for pH mapping of liposomes targeted to tumours.
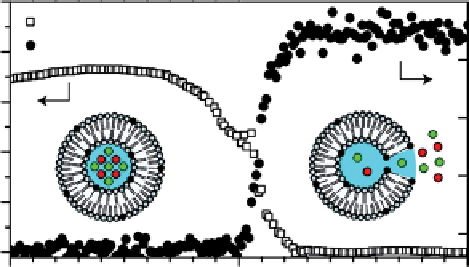
A very interesting temperature-responsive agent that could potentially be applied in the field of imaging-guided drug
delivery has been reported by langereis et al. [78]. localised delivery of anticancer drugs based on liposomal nanocarriers
promises a large therapeutic window with reduced side effects of the treatment. If the drug is released from the liposomal
nanocarrier locally by external stimulation (e.g., causing a temperature increase), therapy is expected to be particularly effec-
tive. In response to a mild hyperthermic treatment (39-42 °C), temperature-sensitive liposomes are known to release the
contents of their inner cavity near the melting temperature (T
m
) of their lipid membrane. Based on this idea, langereis et al.
designed a new type of temperature-responsive liposome useful for both
1
H CEST and
19
F imaging. These liposomes
contained both a chemical shift agent (for lipoCEST detection) as well as a fluorine compound (NH
4
PF
6
, for
19
F detection)
in their lumen. Inside the liposome, the
19
F spectral lines are strongly broadened and not detectable due to fast relaxation
induced by the paramagnetic SR. At the T
m
of the liposomal membrane, the chemical shift agent and the fluorinated
compound are both released. This results in the disappearance of the lipoCEST contrast and a simultaneous appearance of
the
19
F MR signal because it is no longer influenced by the SR (Figure 10.17). Hence, the
19
F signal could be used to quan-
tify the amount of released drug payload, while the CEST signal could measure the local nanocarrier concentration before
the release.
In principle, Gd(III) complexes are not considered for the design of paramagnetic CEST agents for two reasons: (i) They
cause a marked relaxation enhancement of water protons (both T
1
and T
2
) that is detrimental to CEST contrast detection, and
50
1
H lipoCEST
19
F NMR
40
30
20
10
0
300
310
Temeprature /K
320
fIgurE 10.17
1
H CEST effect and
19
F NMR signal intensity of liposomes containing Tm-HPDO3A and NH
4
PF
6
acquired as a function
of temperature, with irradiation field intensity of 4.5 μT and at 7 T. Reproduced with permission from Ref. [78].