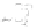Chemistry Reference
In-Depth Information
-
OOC
COO
-
N
N
1
Tm
N
N
-
OOC
CON
B
0
COO
-
2
N
-
OOC
CON
N
Tm
(Δχ)
LIPO
<0
(Δχ)
LIPO
>0
-
OOC
N
COO
-
Tm-1
-
OOC
-
OOC
3
NN
N
Tm-2
Tm
N
N
N
-
OOC
-
OOC
N
N
Tm-3
COO
-
4
N
CONH
-
OOC
Tm-4
Tm
N
CONH
N
COO
-
Tm-5
COO
-
5
O
N
30
20
10
0
Δ
LIPO
-ppm
-10
-20
-30
-40
O
O
CONH
-
OOC
Tm
N
CONH
O
O
N
O
COO
-
fIgurE 10.16
Δ
intralipo
values (25°C) for a series of nonspherical lipoCEST agents encapsulating Tm-HPDO3A and incorporating the
amphiphilic Tm-complexes reported on the left.
incorporating amphiphilic paramagnetic complexes, endowed with the appropriate magnetic susceptibility anisotropy, in the
liposomal membrane. To demonstrate this concept, a number of nonspherical lipoCEST agents with intracavity water proton
frequencies ranging from +30 to −45 ppm have been investigated (Figure 10.16).
This significant separation of the intracavity liposomal water from the resonance of bulk water could drastically reduce
the artefacts in the MRI-CEST images generated by the asymmetry of the bulk water signal and/or the inhomogeneity of the
imaging coil. Furthermore, the extension of the irradiation frequency values could essentially facilitate the setup of imaging
protocols for the visualisation of multiple lipoCEST probes.
A very elegant demonstration showing the relationship between the magnetic field orientation and the chemical shift of
the intravesicular water protons for nonspherical lipoCEST agents has been reported by Burdinski et al. [72] A 100 μm (inner
diameter) capillary, coated with a monolayer of cyclodextrins and capable of hosting liposomal surface-exposed adamantane
moieties, was utilised. Amphiphilic Dy(III) and Tm(III) complexes were incorporated into the liposomal membrane while
aqueous solutions of paramagnetic SRs were encapsulated in the inner cavity. The liposomes were tightly bound to the capil-
lary surface and upon parallel or perpendicular alignment of the capillary with respect to B
0
, their magnetic alignment-
dependent CEST properties were observed. These results showed that the orientation induced by the binding of nonspherical
liposomes to a target surface could be determined by using routine CEST methods. As a corollary, these findings offer
unique opportunities in molecular MRI applications as bound and unbound lipoCEST agents could be easily distinguished
based on the differences in their CEST resonance frequencies. Further enhancement of the magnitude of Δ
int
ralipo
has also
been achieved by encapsulating neutral multimers in the intraliposomal cavity [73].
One of the major advantages of CEST methodology is the possibility of visualising multiple probes in the same MR
imaging voxel. For
in vivo
applications, it is imperative that the CEST agent displays excellent sensitivity and their mobile
protons exhibit significantly different resonance frequencies. Current lipoCEST agents fulfil both requirements; this has
facilitated their translation
in vivo
. The first
ex-vivo
co-localisation of two lipoCEST agents was carried out on a bovine
muscle used as a tissue surrogate [74]. The individual responses of the two agents did not appear to interfere with each other,