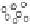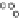Chemistry Reference
In-Depth Information
(ii) the isotropic distribution of the seven unpaired electrons in the f-orbitals prevents these complexes from acting as SRs.
However, when the Gd(III) complexes are entrapped in nonspherical liposomes, they yield a lipoCEST agent analogous to
the above described systems based on other paramagnetic lanthanide ions. Actually, it has been shown that Gd-HPDO3A (a
commercial relaxation agent widely used in the clinical practice) entrapped in a liposome yields a system that works both as
a T
1
/T
2
contrast agent and a CEST agent [79]. As the osmolarity of the suspension was increased by adding NaCl, the lipo-
somes shrunk, thus releasing part of the intraliposomal water to attain the same osmolarity as the outside medium. This
phenomenon was accompanied by a loss of the spherical shape of the vesicles, a change that was clearly detected as a pro-
gressive downfield shift in the resonance of the entrapped water molecules. When the osmolarity of the suspension was in
the range of biological fluids, the shift of the internal water was approximately 7 ppm from bulk water. This increase in the
shift was accompanied by an increase in linewidth that probably accounted for both an enhanced intraliposomal Gd complex
concentration and the consequent increase in R
2
of the encapsulated water protons.
Delli Castelli et al. [80] recently attempted to gain more insight into the understanding of the
in vivo
fate of liposomes
and their payload by comparing contrast changes induced by the presence of a classical relaxation agent with the effect
induced by a CEST agent. liposomes were loaded with the paramagnetic complexes Gd-HPDO3A and Tm-DOTMA in
order to endow the nanovesicles with the characteristic properties of T
1
/T
2
and CEST/T
2
MRI agents, respectively. The para-
magnetically loaded liposomes were injected directly into the tumour (B16 melanoma grafted in mice) where they generated
T
1
, T
2
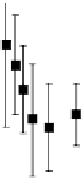
, and CEST contrast in MR images that was quantitatively monitored over time (0-48 h) (Figure 10.18, left). A kinetic
model was devised to fit the experimental multi-contrast data in order to extract the relevant information about the cellular
uptake of the liposomes and the release of their payload (Figure 10.18, right). upon comparing conventional stealth lipo-
somes with pH-sensitive liposomes, it was shown that the latter type differed substantially in the step associated with release
of the drug, which most likely occurred in the endosomal acidic vesicles.
Finally, analogues of lipoCEST agents can be obtained by using di-block copolymer vesicles loaded with paramagnetic
SRs. Block copolymer vesicles have some key advantages over liposomes, such as their lower critical aggregation
concentration and their tunable membrane properties that are controlled by the nature and molecular weight of the hydro-
phobic block [81]. Such vesicles having biocompatible hydrophilic poly(ethylene glycol) (PEG) blocks are well-known for
their long blood circulation time due to reduced opsonisation as well as their water diffusion across the polymer membrane.
T
1
ehh%
CEST %
R
2
50
B
40
D
Cell
A
k
4
k
2
k
3
30
k
1
C
k
5
E
20
F
Extracellular
space
10
0
k
1
k
2
k
3
k
4
k
5
A
Uptake
B
Intra-
vesicles
release
C
Cytosol
release
Esocytosis
E
F
0
1
2
3 4
Time (hours)
5
24 6 8
Wash-out
Blood vessel
Intracellular vesicle
Nucleus
Paramagnetic complex
Liposome
fIgurE 10.18
left: Temporal evolution of the three contrast modes (means and standard deviations of 6 mice) after intratumoural
injection of paramagnetic stealth liposomes. The reported R
2
values refer to data obtained from mice treated with liposomes loaded with
Tm-DOTMA. Right: Schematic representation of the kinetic model used for the analysis of the temporal evolution of the MRI responses
after the administration of paramagnetic liposomes acting as multi-contrast agents. Reproduced with permission from Ref. [80].