Chemistry Reference
In-Depth Information
Off
On
Off-on
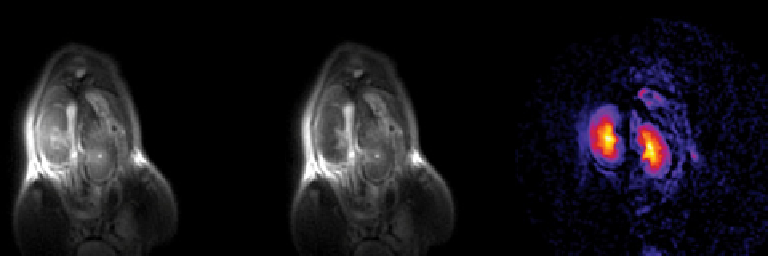
fIgurE 10.12
In vivo
CEST images of mouse kidney after intravenous injection of 1 mmol/kg EuDOTA-(gly)
4
-
using the SWIFT
pulse sequence. Adapted with permission from Ref. [64]. (
See insert for colour representation of the figure.)
)
demonstrated that carbohydrate moieties from endogenous structures such as glycoproteins and glycolipids present in liver
did not give a sufficient background signal to interfere with glucose sensing by the PARACEST agent. In separate experi-
ments, the agent has also been shown to be capable of detecting glucose exported from hepatocytes after hormonal stimulation
of glycogenolysis. It is important to note that the amount of agent used in this perfused tissue experiment was not high enough
to cause a dramatic change in water linewidth due to the T
2exch
contribution described above. This bodes well for eventual
translation of PARACEST-base sensors into the
in vivo
setting as long as they can be used at moderate concentrations.
To take full advantage of the on/off RF activation properties of PARACEST agents
in vivo
, imaging sequences that recap-
ture the T
2
shortening effects induced by the PARACEST agents are required. A potential solution to this problem was high-
lighted by Soesbe et al. [63], who implemented the SWIFT imaging sequence characterised by ultra-short TE (<10 μs) in an
attempt to reclaim the loss in signal due to T
2
exchange. This experiment demonstrated for the first time that the shortened
T
2exch
component can be recaptured and used to detect a water exchange-based PARACEST agent
in vivo
using traditional
on/off CEST imaging (Figure 10.12).
The future of PARACEST agents as biological sensors remains bright because significant progress is being made in the
development of imaging sequences that will allow their detection
in vivo
. The ultimate sensitivity or detection limit of these
agents
in vivo
will also need to be optimised; however, this can be done by fine-tuning the water exchange kinetics of these
agents to make them optimal at 37 °C. One common approach to improve sensitivity is to prepare low molecular weight
polymers of the agents [10, 11] or add multiple copies to a dendrimer [65] or a nanoparticle [12, 15]. Such strategies make
it feasible to lower the detection limits of these novel sensors into the μM range.
10.6
SuPraMolEcular cESt agEntS
As discussed above, the sensitivity issue of CEST agents can be tackled either by exploiting larger
k
ex
values or by designing
systems containing a high number of mobile protons. In an attempt to address the latter parameter, van Zijl and co-workers
explored the use of several diamagnetic macromolecular systems (polyaminoacids, dendrimers, RNA-like polymers) and
found that a CEST effect of approximately 50% could be generated for a 5 μM sample of polyuridylic acid (poly(ru)) [66].
Such a high sensitivity is the result of a high number of irradiated mobile protons (ca. 2000 per polymer). Although these
findings represent an important step in the search for more sensitive CEST agents, it is evident that a much higher improve-
ment could have been realised if such exchangeable proton pools displayed much larger
Δω
values. On this basis, it was
deemed of interest to explore the use of supramolecular adducts between species containing a high number of exchanging
protons and a paramagnetic shift reagent. The major advantage of this system resides in the fact that the resonance frequency
of the mobile protons, upon interacting with the paramagnetic agent, is shifted away from its diamagnetic position, thus the
observed CEST effect is not affected by the contribution of protons belonging to endogenous molecules. Furthermore, the
marked increase in
Δω
would also allow the exploitation of faster proton exchanging systems (larger
k
ex
).
As a model system to prove the efficacy of this approach, the supramolecular adduct formed by the cationic polypeptide
poly-l-arginine (53.5 kDa) and the negatively charged lanthanide complex Tm(HDOTP)
4-
, whose ability to act as NMR shift
reagent for cationic species is well documented, was investigated [8, 67, 68]. In the absence of the shift reagent, the exchange
rate of the mobile guanidine protons of the polypeptide at pH 7.4 and 312 K was so fast that no CEST effect could be
observed (Figure 10.13, open squares). However, upon the addition of Tm(HDOTP)
4-
to the poly-l-arginine solution, a
remarkable transfer of saturated magnetisation was observed around 20 to 30 ppm downfield of the water resonance
(Figure 10.13, filled squares), which was an unambiguous indication of the formation of a tightly associated ion pair. It is