Chemistry Reference
In-Depth Information
ratiometric imaging to obtain a direct readout of pH. The pH of five aqueous phantom samples as determined by imaging
(Figure 10.8, bottom right) were in perfect agreement with those measured directly by use of a pH electrode.
The accessibility of bulk water molecules to the coordination sphere of a lanthanide cation in PARACEST complexes is
another factor that affects
k
ex
. This can be exploited in the design of responsive agents. using this approach, sensors for
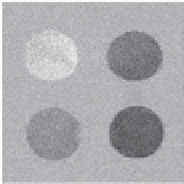
glucose [38, 42], Zn(II) [37], and Ca(II) [55], have been designed and evaluated. In the case of the glucose sensor, water
exchange was somewhat too fast to produce a CEST signal from the exchanging water molecule, but CEST was turned on
upon binding of glucose to the two phenylboronate moieties situated directly above the exchanging water molecule
(Figure 10.9, top). This binding event served to slow water exchange by hindering access of bulk water molecules to the
water coordination site, thus resulting in an increase in the CEST intensity. A similar strategy was used in the design of a
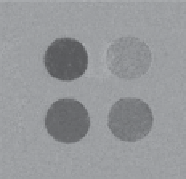
Zn
2+
sensor, but, in this case, binding of a single Zn
2+
ion directly above the water coordination site had the opposite effect,
where
k
ex
was found to increase upon Zn
2+
binding due to catalysis of proton exchange by a proximate Zn
2+
-coordinated OH
group (Figure 10.9, bottom). This resulted in switching the CEST signal 'off' when the Eu
3+
complex was fully bound with
Zn
2+
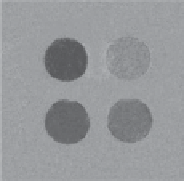
. The Ca
2+
sensor also registered a reduction in CEST intensity upon Ca
2+
binding but this probe was not selective for
Ca
2+
as it was able to elicit a similar response in the presence of Mg
2+
. Additionally, PARACEST agents that detect lactate
[44] and a number of anions [49, 50, 56] have also been reported.
Chemical modification of a PARACEST agent by the action of an enzyme or a specific reaction with another substance
can result in a new entity with different CEST properties. This approach has been used for the detection of caspase-3 [47]
and esterase [45] enzyme activity by following a decrease in the CEST signal from a slowly exchanging amide proton in the
initial sensor as well as the appearance of a new CEST signal from the more rapidly exchanging amine protons. A similar
approach was also reported for the detection of nitric oxide [46].
One of the major challenges in the development of PARACEST agents is that conventional solution-phase synthesis of
these agents is time consuming and generally limits studies to one agent at a time. In an effort to overcome this limitation,
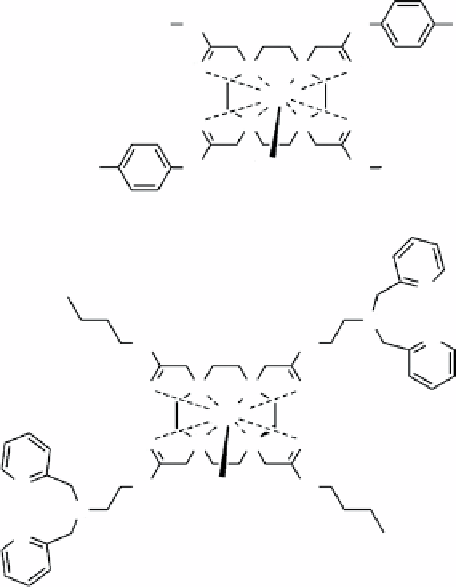
Napolitano et al. [35] recently reported a rapid, convenient on-bead synthesis of a library of 80 different PARACEST agents
that differed only in the chemical identity of three amide side-chain groups. Without removing the complexes from beads,
CEST imaging was performed to quickly identify those agents (and side-chains) that exhibited the most favourable water
exchange kinetics for CEST (Figure 10.10).
0
10
NH
NH
B(OH)
2
O
NN
O
Eu
3+
5
20
O
O
NN
O
H
2
O
(HO)
2
B
NH
NH
N
N
N
NH
NH
Control Zn
II
0
20
O
NN
O
Mg
II
Ca
II
5
10
Eu
3+
O
O
NN
O
N
H
2
O
NH
NH
N
N
fIgurE 10.9
Top: Structure of glucose-responsive PARACEST agent (left), and CEST images of phantoms containing PARACEST
agent and different concentrations of glucose (right). Reproduced with permission from [42]. Bottom: Structure of zinc-responsive
PARACEST agent (left), CEST images of phantoms containing PARACEST agent and different concentrations of Zn
2+
(centre), and
PARACEST agent with different metal ions (right). Reproduced with permission from Ref. [37].