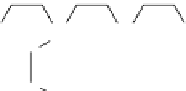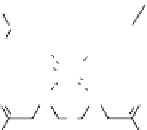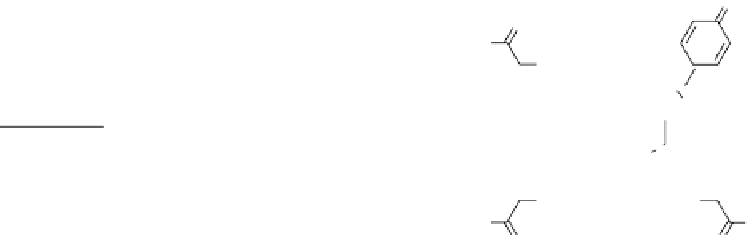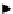Chemistry Reference
In-Depth Information
3
1.0
-
O
0.8
*
2
pH 7.31 37°C
pH 7.31 20°C
0.6
O
NN
O
H
Yb
3
+
0.4
O
NN
O
1
0.2
-
O
O
-
0
0.0
-200
-100
0
ppm
100
200
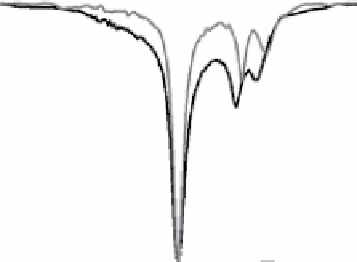
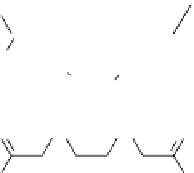
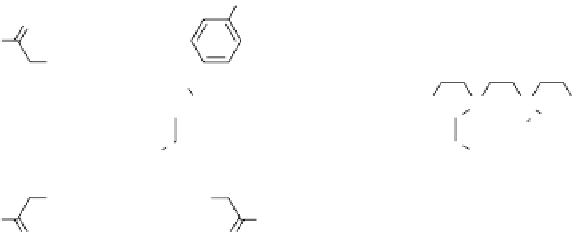
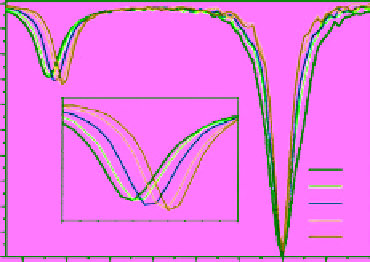
fIgurE 10.7
left: Chemical structure of Yb(III)-HPDO3A showing exchangeable -OH proton. Centre: CEST spectra of Yb(III)-
HPDO3A at 20°C and 37°C showing the -OH exchange peaks of both isomers in solution. Right: Ratiometric CEST images of a phantom
containing 14 capillaries of Yb(III) HPDO3A. Images were acquired after irradiation at 71 ppm and 99 ppm and at pH values ranging from
5.2 to 8.8. Adapted with permission from Ref. [52].
O
-
OH
O
O
O
O
-O
-O
-O
NH
NH
NH
O
-
NN
O
NN
O
NN
O
-H
+
O
O
Eu
3+
Eu
3+
Eu
3+
N
N
N
O
N
O
O
N
O
O
N
O
H
2
O
HN
H
2
O
HN
H
2
O
HN
NH
NH
NH
O
-
O
-
O
-
-O
-O
-O
O
O
O
O
O
O
100
90
80
70
60
7.2
7.7
7.5
7.4
W
7.0
100
95
90
85
80
75
70
65
50
6.7
40
30
20
10
0
7.6
7.2
6.8
6.4
6.0
6.4
pH 7.6
pH 6.0
6.0
6.0
60
54
Saturation offset (ppm)
57
51
48
45
5.7
6.4
60
50
40 30
Saturation offset (ppm)
20
10
0
-10
-20
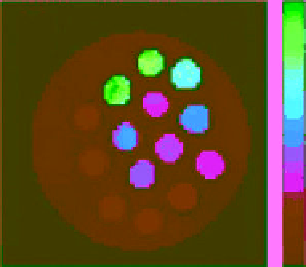
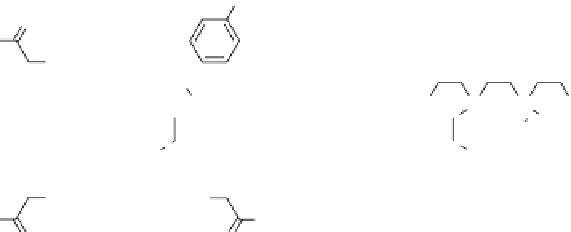
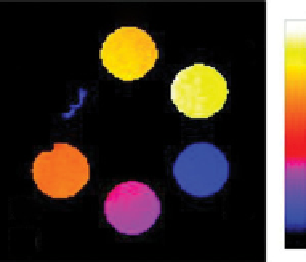
fIgurE 10.8
Top: Change in chemical structure of pH-responsive PARACEST agent as pH is increased. Bottom: pH dependence of
CEST spectra showing changes in chemical shift (left), and CEST pH images of a phantom containing water (w) and PARACEST agent
after activation at 54 ppm and 47 ppm (right). Adapted with permission from Ref. [41]. (
See insert for colour representation of the figure.)
)
this is an overall weaker ligand field around the Eu
3+
ion and therefore, a smaller Δω. As the pH is increased, the phenolic
group loses a proton and the negative charge on the phenolate anion is delocalised through the ligand π-system onto the
ketone oxygen donor atom. This results in greater electron density on the ketone oxygen, an overall stronger ligand field for
the Eu
3+
ion, and an increase in Δω for the exchanging water molecule (Figure 10.8, top). The difference in Δω between the
high and low pH extremes was surprisingly large, ~7 ppm (Figure 10.8, bottom left), making it feasible once again to use