Chemistry Reference
In-Depth Information
MRI contrast produced by PARACEST agents can be generated via exchange of highly shifted lanthanide-bound water
molecules (H
2
O) [10, 11, 15, 32-42], amide protons (-NH) [43-48], or hydroxyl protons (-OH) [49-52], depending upon
which lanthanide ion is used. Because one can chemically modify one or more ligand side chains and alter the water
exchange kinetics of a system rather easily, the development of responsive or 'smart' PARACEST agents has become a focus
of many research laboratories involved in the design of novel MRI contrast agents.
10.4
rESPonSIvE ParacESt agEntS
Since
k
ex
and ∆ω are both quite temperature sensitive (with
k
ex
typically increasing and ∆ω decreasing with temperature), the
magnitude of the CEST signal also happens to be temperature sensitive, sometimes in unexpected ways. This makes PARACEST
agents particularly interesting for monitoring temperature in biological systems. Because the hyperfine shift induced by a para-
magnetic lanthanide ion is always inversely proportional to temperature (can have both T
1
and T
2
components), one can quite
easily and predictably obtain temperature maps [33, 53] by simply measuring ∆ω and using a calibration curve.
The rate of proton exchange (
k
ex
) for -NH systems is strongly pH dependent. Above pH ~5, base catalysis of -NH
exchange becomes dominant, resulting in an acceleration in
k
ex
. Because the magnitude of the CEST effect is strongly influ-
enced by
k
ex
, changes in the CEST spectrum can be used to assess differences in pH. This concept was elegantly demonstrated
by Aime et al. where a mixture of Eu
3+
and Yb
3+
DOTA-(gly)
4
-
and a ratiometric imaging approach was used to estimate
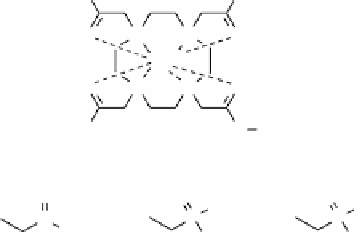
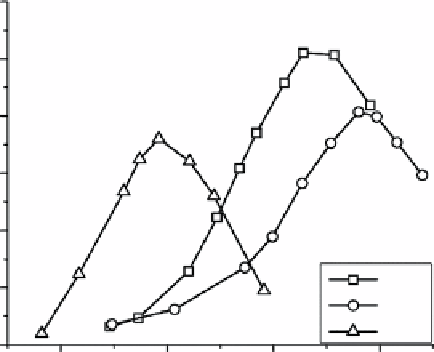
pH [43, 54]. More recently, Opina et al. used a series of different Yb
3+
DOTA-tetraamide complexes to show that the range of
pH sensitivity could be further adjusted by altering the electrostatic properties of the amide substituents (Figure 10.6) [19].
The rate of proton exchange from -OH groups is also pH dependent. For DIACEST compounds, this dependence is not
terribly useful for measuring pH in the physiological range, but for PARACEST systems involving an -OH group directly
bound to a lanthanide ion, the pH sensitivity of -OH proton exchange once again becomes interesting. In a recent report, the
CEST spectrum of Yb(III)HPDO3A, an analogue of GdHP-DO3A(Prohance), shows two highly shifted -OH exchange
peaks (at 99 ppm and 71 ppm) reflecting the presence of either two different stereoisomers or coordination isomers in solu-
tion [52]. Interestingly, the exchange rates for these two -OH protons differ quite substantially, and this feature allows
construction of a ratiometric calibration curve to evaluate solution pH (Figure 10.7).
Furthermore, it has been demonstrated that the chemical shift between the lanthanide-bound water and bulk water (∆ω)
in various lnDOTA-tetraamide complexes can be altered by varying the electron density either on one [36] or all four [40]
of the amide substituents. This observation led to the development of a pH sensor [41] that responds to changes in pH by a
change in chemical shift of the water exchange peak position rather than a change in CEST intensity. This feature is highly
advantageous because the sensor readout (in this case, chemical shift) is independent of concentration. To accomplish this,
one of the amide side chains of a DOTA-tetraglycinate was replaced with a ketone moiety having an oxygen ligand donor
atom in conjugation with a phenolic group. At pH values below the pK
a
of the phenol group (in this case, about 6.7), the
ligand donor is essentially an isolated ketone oxygen atom with relatively poor electron donating capabilities. The result of
60
R-
N
H
H
N-
R
50
O
N
N
O
Yb
3+
O
N
N
O
40
H
N
R
R-
N
H
30
O
O
O
O
-
O
-
20
OEt
P
P
C
R=
O
-
OEt
Yb-1
10
Yb-2
Yb-3
(Yb-1)
(Yb-2)
(Yb-3)
0
6
7
8
9
pH
fIgurE 10.6
left: Chemical structures of Yb
3+
DOTA-tetraamide complexes with differing amide substituents. Right: Plots of CEST
magnitude
versus
pH for each complex. Adapted with permission from Ref. [19].