Chemistry Reference
In-Depth Information
O
H
N
H
COCH
3
*
H
O
O
O
O
O
O
*
-
OOC
-
O
3
SO
n
O
H
δ = -1.0 ppm
δ = +1.0 ppm
Difference image
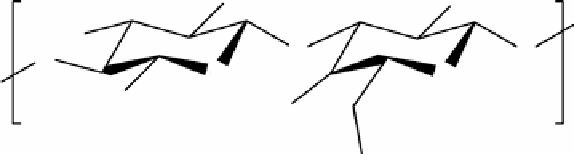
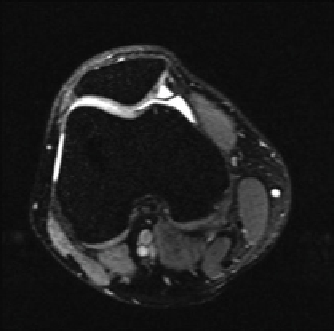
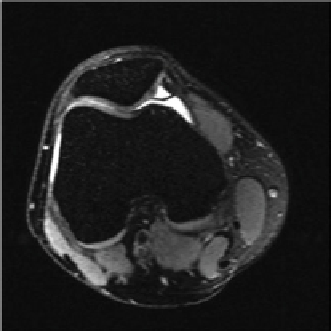
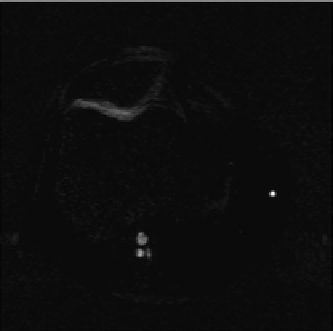
fIgurE 10.5
Top: Structure of chondroitin sulphate, a basic unit of GAG. Bottom: CEST images of a human patella
in vivo
with irra-
diation at −1.0 ppm (left), and at 1.0 ppm (centre). The difference image outlined a clear demarcation of a cartilage lesion on the medial
facet (right). Reproduced with permission from Ref. [27].
10.3
ParaMagnEtIc chEMIcal ExchangE SaturatIon tranSfEr (ParacESt) agEntS
Paramagnetic CEST agents represent another class of chemical exchange systems that offer some potential advantages
and disadvantages over DIACEST agents. The most common of these agents are derived from paramagnetic lanthanide ion
complexes with either exchangeable -NH protons or lanthanide ion-bound water molecules. The major advantage paramag-
netic agents have over their diamagnetic counterparts is a much larger frequency difference (Δω) between the exchanging
proton (or water molecule) with respect to the bulk water frequency. This large frequency difference allows much easier RF
saturation of the paramagnetically shifted proton without indirect partial saturation of bulk water protons. Thus, the saturation
selectivity improves and there is less need to perform an asymmetry analysis (difference between saturation at ± offset
frequencies on each side of the water peak). The larger frequency difference also permits the use of faster exchanging species
without approaching the fast exchange limit. This means that unlike a typical DIACEST compound containing a -NH proton
exchanging with water at a rate of 500-1000 s
-1
, one can envision using systems that undergo exchange much more frequently,
perhaps as fast as 5000-10,000 s
-1
. unfortunately, one major disadvantage of such faster exchanging systems is that they
require more RF power for saturation, which could potentially result in tissue heating, depending upon coil efficiency and
other factors. Thus, paramagnetic systems that combine large hyperfine shifts in chemical sites with moderate-to-slow proton
exchange groups (-NH, -OH, or bound water molecules) offer the advantages of both DIACEST and PARACEST. Slow proton
or water molecule exchanging systems will likely be a requirement of those systems that are ultimately moved forward toward
clinical use. This requirement has increased interest in the chemical community for identifying lanthanide complexes that have
water exchange kinetics two to three orders of magnitude slower than their DOTA and DTPA counterparts [28-30].
The slower water exchange properties of lanthanide complexes formed with DOTA-tetraamide ligands originates from
the relatively poor electron-donor ability of the amide oxygen donors compared to carboxylate oxygen donors. This
condition renders the lanthanide ion more electron-deficient and increases its affinity for any directly coordinated water
molecule. The observation that the exchange rate of water in various Eu
3+
complexes is the slowest amongst all other lan-
thanide complexes of the same ligand [31] has led to the conclusion that the EuDOTA-tetraamide complexes have the best,
although not optimal, water exchange kinetics for
in vivo
use. Hence, the majority of water exchange PARACEST agents
reported to date have been modifications of the basic Eu(III) DOTA-tetraamide structure. These symmetric complexes typically
have Eu
3+
-bound water resonances near 50 ppm, depending upon temperature [32].