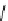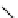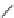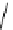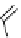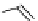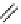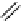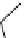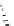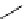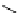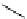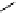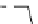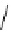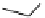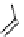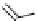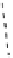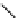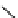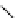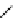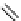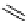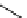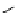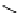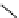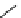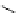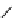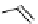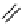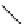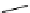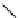Chemistry Reference
In-Depth Information
Contrast agents whose relaxivities are dependent on pH are highly sought after, owing to the observed difference in extra-
cellular pH found within tumours (ca. 6.8 − 6.9) with respect to normal tissue (ca. 7.4) due to excess lactic acid production
in tumour cells. A critical MRI parameter, for example, q, τ
r
, or τ
m
, must be substantially affected by pH in the proposed
system. The image intensity will depend upon both the relaxivity and the concentration of the contrast agent and therefore
non-uniform dispersion of the agent creates a challenge. This can be surmounted, for example, by taking the difference of
images obtained using two contrast agents with differing pH-relaxivity functions but similar biodistributions administered
in quick succession.
Parker and co-workers investigated dO3A-based systems incorporating sulfonamide pendant arms with various
p
-aryl
groups and β-carboxyalkyl substituents α to the ring nitrogens (to disfavour the binding of endogenous anions, as shown for
[gd.
RRR
-gadO3A]
3-
above). The hydration number in these systems is approximately zero in alkaline media and greater
than one in acidic media, corresponding to ligation to gd and protonation of the sulfonamide nitrogen respectively, leading
to relaxivity enhancement with decreasing pH [87, 88]. The change in relaxivity of the gd(III) complexes in the biologically
relevant pH range 6.8 − 7.4 reached a maximum of 48% (for gd.Sulfon-gadO3A, Figure 8.8) in a human serum solution,
with protein binding enhancing the effect with respect to a simulated extracellular anion background. The pH response
of the complexes depends heavily on structure, with a closely related structure showing a change in serum solution of 15%,
thus providing a means of circumventing the concentration problem. The pH-sensitive sulfonamide unit was utilised in ratio-
metric measurement through the application of
19
F/
1
H MRI methods to self-assembled systems based on cyclodextrins (see
above) [81]. A good example of the effect of pH on τ
r
is found with the macromolecular [gd(dO3ASQ)]
30
-Orn
114
complex
studied by Aime et al. (Figure 8.8) [89]. Thirty gd.dO3A units are linked to a polyornithine chain via squaric acid moieties,
and a linear increase in relaxivity from 23 to 32 mM
-1
s
-1
ongoing from pH 4 to 8 is measured. This response is explained by
considering the structural changes occurring with pH. At acidic pH, protonated amino groups repel one another to give a
highly flexible structure with mobile gd.dO3A units. As the pH rises and amino groups become deprotonated, hydrogen
bonding within the peptide backbone causes a rigidification of the structure associated with an increase in τ
r
, confirmed by
assessment of the NMRd profile. These macromolecular complexes were used again in an investigation into a ratiometric
approach to the concentration problem. In the case where one of T
1
or T
2
is dependent on the biological variable of choice,
but not the other, the ratio T
1
/T
2
becomes independent of local concentration of contrast agent. This concept was successfully
H
2
N
O
O
N
O
HO
2
C
N
O
H
S
30
O
N
N
84
O
CF
3
Gd
O
N
N
O
NH
O
O
CO
2
H
O
O
O
N
N
O
Gd
O
N
N
HO
2
C
O
O
O
Gd.sulfon-gaDO3A
[Gd(DO3ASQ)]
30
-Orn
114
O
O
O
O
O
O
N
N
N
N
O
O
Gd
Gd
CO
-
O
O
CO
-
N
NN
N
CO
-
CO
-
O
O
N
N
O
O
O
O
Gd.DOPTA
fIgure 8.8
Structures of pH and pCa responsive contrast agents.