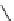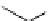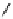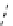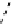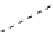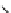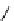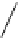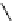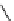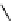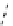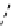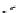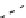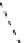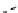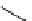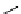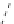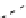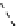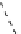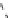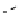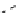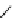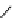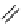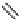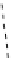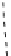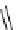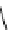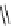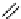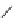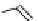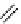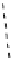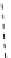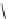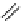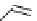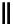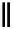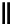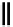Chemistry Reference
In-Depth Information
(a)
(b)
O
HO
O
O
N
N
N
N
O
Gd
O
O
O
O
N
N
N
O
O
O
O
O
N
N
O
O
O
N
Gd
O
OH
HO
N
N
N
HO
O
O
O
O
O
O
HO
fIgure 8.9
Variations in structure between calcium and zinc responsive contrast agents (a and b respectively).
O
O
OH
2
HO
O
OH
2
OH
O
O
O
H
O
H
O
N
N
O
O
N
β-galactosidase
OH
2
N
N
Gd
N
Gd
OH
O
OH
O
Gd
N
N
O
N
O
O
N
N
O
N
O
O
O
O
O
O
Steric bulk of galactose unit
and interaction between Gd
3+
and sugar hydroxyls prevents
close approach of water
i.e., LOW relaxivity
Once the sugar has been cleaved,
there is easy access for water molecules in both q=1 and q=2
forms of the complex
i.e., HIGH relaxivity
fIgure 8.10
The mechanism of action of a contrast agent responsive to the enzyme galactosidase. We have represented the cyclen
backbone structure to enable comparison with the representation of dOTA structures in Figure 8.3.
demonstrated for a [gd(dO3ASQ)]
33
-Orn
205
complex, whose T
1
and T
2
show differing pH dependencies, especially at higher
fields [90].
Another highly desirable target is a response to biologically relevant metal ions [91]. For example, calcium is important
as a signalling ion in biology, especially in the brain, where changes in Ca
2+
concentration can be indicative of neural
function. The first gd-based contrast agent to display relaxivity modulation selective for Ca
2+
was presented by Meade and
co-workers and is known as gd.dOPTA (Figure 8.8) [92]. The design combines two gd.dO3A units with a BAPTA core via
propyl linkers, the latter being a well-known selective binder of calcium (over, e.g., H
+
and Mg
2+
). In the absence of calcium
ions, the acetate groups of the BAPTA core interact with the gd centres and inhibit close approach of water. Addition of
calcium causes a rearrangement of the ligand with the BAPTA now binding calcium, thus allowing access of water to the gd
sites and resulting in an increase in relaxivity of approximately 80% (in the [Ca
2+
] range 0.1 to 10 μM). Further studies into
the mechanism of the response involved luminescence measurements of the analogous terbium complex. Assessment of the
luminescence lifetimes in H
2
O and d
2
O in the absence and presence of Ca
2+
revealed an increase in the number of bound
water molecules from 0.47 to 1.05, consistent with the proposed mechanism [93].
Mishra et al. have investigated Ca
2+
and Zn
2+
-binding gadolinium complexes of related structures (A and B respectively
in Figure 8.9), with eight and six coordinate binding environments for the guest metal respectively [94]. As with Meade's
complex, the mechanism of increased relaxivity upon Ca
2+
/Zn
2+
binding is also related to the hydration state of the gd(III)
ion. Luminescence studies of the corresponding europium complexes show an increase in q from about 0.4 to 1 upon ion
binding, with a change in coordination environment at Eu(III) indicated by a change in the ratio of the hypersensitive ΔJ = 2
transition to the ΔJ =1 transition. The reversibility of the response was demonstrated by competitive binding of the divalent
metal ions to EdTA to give restoration of the initial relaxivity values. Studies in mouse serum reveal approximately 30 − 40%