Chemistry Reference
In-Depth Information
N
O
O
O
N
O
O
O
N
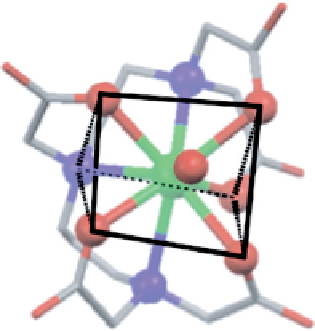
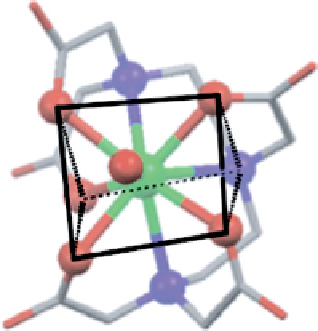
fIgure 8.2
The two enantiomers of dy.dTPA found in the X-ray structure [14] viewed approximately along the H
2
O−dy bond, with
donor atoms and the central metal shown as spheres and trigonal prisms superimposed on the atoms.
(Omniscan
®
), [gd.HP-dO3A] (Prohance
®
), [gd.dO3A-butrol] (gadovist) and [gd.MS-325]
2-
(Angiomark
®
) [8-13]. The
ninth coordination site in these complexes is occupied by a water molecule (see below). dTPA and dOTA bind to trivalent
lanthanide ions through three or four amine nitrogen atoms and five or four carboxylate oxygen atoms respectively. In the
body, these complexes behave similarly, presenting a hydrophilic surface where the ligand surrounds the toxic gd(III) ion,
but allows access to a single water molecule.
Closer examination of the solid-state structures reveals differences. The most favourable geometries for nine-coordinate
lanthanide complexes are the tricapped trigonal prism (TTP) and the monocapped square anti-prism (SAP). In the solid state,
dTPA and its derivatives and analogues tend to adopt a distorted TTP geometry [14]. There are two possible ways in which
the acyclic dTPA can encase the lanthanide ion, giving two enantiomeric 'wrapping' isomers and making the central nitrogen
chiral. This can be seen in Figure 8.2, where both enantiomers of the complex are shown with trigonal prisms superimposed
upon the atoms. It is also clear from this figure how close such geometries come to being rationalised by a distorted square
anti-prism geometry (although this is not shown explicitly, it is clear that the upper plane of oxygen atoms resembles a
square, while the N
3
O donor set is distorted from square geometry). Clearly, all possible isomers are broadly comparable,
meaning that interconversion between them should be facile (and that ligand geometry is the ultimate arbiter of the observed
structure). In solution, interconversion between the two enantiomers is achieved by a rearrangement of the acetate arms and
a simultaneous flip between staggered conformations of the diethylenetriamine backbone. Proton NMR studies of the
yb(III), Eu(III), and Pr(III) complexes reveal rapid exchange of isomers at high temperatures, with 18 resonances coalescing
to nine with increasing temperature [15]. The activation barriers to exchange between these isomers are 49.4, 55.4, and
56.5 kJ mol
-1
for yb(III), Eu(III), and Pr(III) complexes, respectively, and reflect the generally flexible nature of the struc-
ture. Flexibility in this system also accounts for the limited kinetic stability of the lanthanide complexes.
In derivatives of dTPA, substitution of acetate arms for other donor groups leads to increased numbers of stereoisomers.
When the diethylenetriamine backbone of dTPA is substituted, for example in MS-325, the chiral centre introduced in
addition to the chirality at the central nitrogen and the wrapping isomers generate eight possible stereoisomers, but also act
as a chiral auxiliary - favouring the formation of some isomers over others and increasing the rigidity of the backbone. By
contrast, in bisamide derivatives of dTPA, such as dTPA-BMA, all three nitrogens are chiral, and there are four possible
diastereomers (
cis
,
trans
,
syn,
and
anti
), which can each exist as two wrapping isomers, giving a total of eight isomers. In
such systems, the reduction in charge that results from replacing two carboxylate donors with uncharged carboxamide
donors results in a reduction in both the thermodynamic and kinetic stability of such complexes. We will deal with this issue
in greater detail later in this chapter.
In the solid state, lanthanide dOTA complexes and derivatives display common features [16]. The four nitrogen donors
form the basal plane of a square anti-prism with four coplanar oxygen donors making up the opposite and parallel face of
the prism. The twist angle between N
4
and O
4
planes defines two possible geometries: a square anti-prism (SAP) with a twist
angle of about 40
°
or a twisted SAP (TSAP) with a twist angle of about 30
°
(Figure 8.3). The metal ion is shifted from the
centre of the prism toward the O
4
plane [17, 18], probably due to differing donor atom affinities. In the solid-state, [gd.
dOTA]
-
and isostructural Eu(III), Lu(III), y(III), and Ho(III) complexes [19-22], have the expected N
4
O
4
ligand coordination
with a capping water molecule. The twist angle in these complexes is 39°, indicative of a SAP geometry. By contrast, the
La(III) complex of dOTA forms a carboxylate bridged chain structure with TSAP geometry with a twist angle of 22°.
gd.HP-dO3A and the isostructural y(III) complex crystallise with two independent molecules in the unit cell, one with SAP