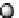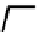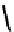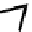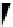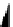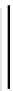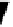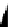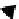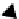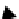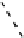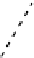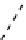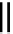Chemistry Reference
In-Depth Information
Twisted square anti-prism
Δ (δδδδ)
Square anti-prism
Λ (δδδδ)
Arm
rotation
Ring
inversion
Ring
inversion
Arm
rotation
Δ (λλλλ)
Square anti-prism
Λ (λλλλ)
Twisted square anti-prism
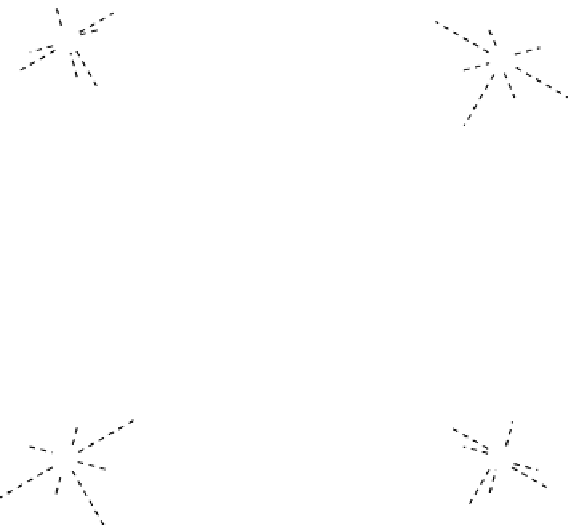
fIgure 8.3
Schematic diagram showing the four possible stereoisomers of lanthanide dOTA complexes.
and one with TSAP geometry [23, 24]. gd.dO3A-butrol crystallises as a carboxylate dimer with TSAP geometry [25].
Study of Figures 8.2 and 8.3 reveals considerable similarity between dTPA and dOTA derivatives when viewed from above
the carboxylate plane: The key to ensuring stability in both kinds of complex lies in maximising the barrier to interconver-
sion between closely related structures.
As we will see as this chapter develops, complexes with macrocyclic ligands generally offer higher degrees of kinetic
stability in comparison with multidentate ligands, particularly for octadentate ligand systems, owing to the increased pre-
organisation of the host molecules and the greater barriers to conformational change conferred by the cyclic nature of their
backbones. Unlike dTPA derivatives, backbone functionalisation can profoundly reduce the stability of lanthanide
complexes. This is particularly true if the backbone is rigidified to the point where the macrocycle becomes sufficiently
inflexible to prevent formation of an appropriate conformation in the complex. Several examples of such systems have been
noted where aryl substituents are appended to a cyclen backbone (e.g., Ph-dOTA, Figure 8.4): In these systems, kinetically
unstable lanthanide complexes form that have no practical use as contrast agents [26].
Lanthanide complexes with dOTA and its derivatives exist as four possible diastereomers (two pairs of enantiomers)
resulting from two independent elements of chirality associated with the macrocyclic ring conformation (λλλλ and δδδδ)
and the spatial arrangement of the acetate arms (Λ and Δ), as illustrated in Figure 8.3. In solution, isomers can interconvert
by ring inversion or acetate arm rotation, with a single process converting SAP and TSAP geometries and two successive
processes exchanging enantiomers. The activation energy barrier to such interconversion is relatively high, and such species
are commonly in slow exchange for octadentate cyclen derivatives. By contrast, heptadentate ligands such as dO3A have
a much lower energy barrier to arm rotation, and exchange between the SAP and TSAP forms can be fast on the NMR
timescale [27].