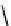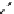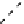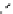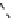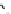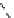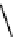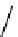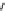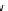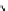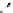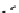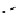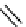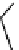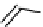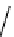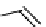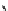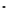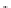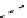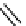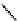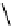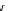Chemistry Reference
In-Depth Information
8.2
gadoLInIum compLexes as mrI contrast agents
In the search for paramagnetic species that can increase the relaxation rates of water protons, gadolinium, with its seven
unpaired electrons, presents itself as a natural choice. Also importantly, its isotropic electronic distribution (symmetric
8
S
ground state) confers relatively slow electronic relaxation rates and gives rise to no paramagnetically induced chemical shift.
The dipolar shift induced by the presence of a lanthanide cation is relatively small, and rapid exchange of bound solvent with
bulk gives rise to a single peak for which the weighted average chemical shift is indistinguishable from bulk water under the
conditions of an MRI experiment. These properties, combined with the chemical stability of gadolinium(III), make gado-
linium contrast agents ideal for the purposes of imaging - provided that issues of toxicity of the free metal can be dealt with
by encapsulation into stable complexes (a topic that we will discuss in detail later in this chapter).
Europium(II) complexes would also be expected to give rise to similar MRI properties. Indeed, they combine more rapid
water exchange at the metal centre (as a consequence of the reduced charge on the lanthanide) with longer electronic relax-
ation times than their gadolinium analogues - meaning that they should be good candidates for use in generating MRI con-
trast [3]. However, despite the fact that some europium(II) species can be isolated in water and despite considerable effort
from a section of the MRI contrast community [4], no chelates have been identified in which europium(II) is sufficiently
stable for clinical application.
For use
in vivo
, the toxicity of the contrast agent is a vital consideration [5, 6]. Toxicity in the free gadolinium(III) ion
results, for example, from having similar size to calcium(II) ions, disrupting Ca
2+
-mediated signalling processes within the
body, and also through the effects of colloidal precipitate formation and accretion in membranes [7]. Thus, gd(III) must be
packaged in such a way as to enhance MRI images but not present a toxic hazard. This is achieved through complexation
with appropriate polydentate ligands. gd(III) complexes need to have both high thermodynamic stability and kinetic inert-
ness whereby they remain intact for the duration of their residence within the patient. Because bonding to lanthanide ions is
primarily ionic in nature, due to the lack of participation of the contracted 4f orbitals in bonding, sufficiently stable complexes
form only with charged polydentate ligands or with macrocyclic ligands bearing pendant donor groups.
The eight-coordinate ligands dTPA and dOTA and their derivatives have become almost ubiquitous in this field, with
several in clinical use (Figure 8.1), including [gd.dOTA]
-
(dotarem®), [gd.dTPA]
2-
(Magnevist
®
), [gd.dTPA-BMA]
O
O
O
O
O
O
O
O
O
N
N
N
N
O
NH
O
Gd
O
N
N
N
N
N
N
Gd
Gd
O
HN
O
O
O
O
O
O
O
O
O
O
O
O
O
[Gd.DOTA]
-
[Gd.DTPA]
2-
Gd.DTPA-BMA
O
O
OH
O
O
O
O
OH
OH
O
P
N
N
N
N
O
O
O
OH
OH
N
O
Gd
Gd
O
O
N
N
N
N
N
N
Gd
O
O
O
O
O
O
O
O
O
O
O
O
[Gd.MS-325]
2-
Gd.HP-DO3A
Gd.DO3A-butrol
fIgure 8.1
Widely studied MRI contrast agents. gadolinium complexes of dOTA, dTPA, dTPA-BMA, HP-dO3A, dO3A-butrol,
and MS-325 are all in clinical use [8-13].