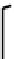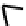Chemistry Reference
In-Depth Information
attached to a DOTA-PEG ligand. This was then labelled with
125
I,
111
In, and
64
Cu, and the biodistributions investigated by
64
Cu PET in athymic mice with xenografted colon tumours showed good tumour and low kidney uptakes [49].
A DOTA-GlyGlu-Cyc lactam bridged cyclised α-melanocyte stimulating hormone peptide was radiolabelled with
67
Ga, which
allowed visualisation of primary and metastatic melanoma, showing potential as a theranostic agent if an analogous DOTA
conjugate was also used to coordinate a therapeutic radionuclide such as
90
y [50]. The first report of effectively synthesised lac-
tam bridge-cyclised alpha-mSH peptides involved In
111
. This displayed high
in vivo
and
in vitro
stability, and melanoma metas-
tases were clearly visualised using SPECT. This procedure confirmed the effectiveness of lactam bridge-cyclised alpha-mSH
peptides for diagnostic imaging of melanoma. Whole-body SPECT imaging revealed that
67
Ga-DOTA-GlyGlu-CycmSH exhib-
ited a rapid tumour uptake, reaching its peak of 12.93% +/- 1.63 two hours after injection. Body clearance was rapid with 82%
of the injected radioactivity being cleared through the urinary system. Normal organ uptakes were low, an exception being the
kidneys, which still retained high amounts of
67
Ga after 2 hours (23.94% +/- 7.15). Furthermore, a DOTA-PEG4-BN bombesin
analogue that was labelled with
67/68
Ga and
177
lu showed good tumour uptake in PC-3 xenografted nude mice, where
67/68
Ga can
act as a diagnostic radionuclide and
177
lu as a therapeutic [51].
yoshimoto et al. radiolabelled DOTA-c(RGDfK) with
111
In and
90
y, which showed high tumour uptake due to specificity
for α
v
β
3
integrin and therefore promise as a theranostic pair [52]. DOTA-neurotensin (DOTA-NT) analogues were designed
by Gruaz-Guyon et al. for targeted radiotherapy with
90
y or
177
lu and PET or SPECT imaging labelled with
68
Ga or
111
In [53].
The
111
In DOTA-NT conjugate showed higher tumour and renal uptake (by SPECT) than the
68
Ga DOTA-NT conjugate
imaged by PET, with very low background in tissues with the exception of the kidney. yttrium displayed greater affinity than
indium for DOTA-NT, confirming the potential for tumour targeting as a radiotherapeutic. Recently, cyclic peptides have
also been applied for combined near infrared fluorescence and SPECT imaging of tumours, allowing unambiguous visuali-
sation of the tumour by both modalities [54].
Several DOTA-conjugated gonadotropin-releasing hormone Receptor-Targeting (GnRH) peptides have been designed
and synthesised. The DOTA group was conjugated to the epsilon or
alpha
-amino group of d-lysine or the epsilon amino
group of l-lysine via an aminohexanoic acid (Ahx) linker to generate the corresponding DOTA-Ahx-(d-lys
6
-GnRH) con-
jugates. The radiopharmaceutical
111
In-DOTA-Ahx-(d-lys
6
-GnRH) (whereby the radiometal was attached at the epsilon
amino group of d-lysine) was synthesised (95% yield) and characterised as a possible agent for imaging prostate cancer. In
order to ensure maximum binding affinities, both the N and C termini of the peptide chain need to be preserved [55]. The
introduction of Ahx was used to increase the lipophilicity of the attached d-lys
6
-GnRH peptide to favour receptor binding
[55]. The chelator DOTA was used because of its known thermodynamic stability and that its conjugation to the epsilon
amino group of d-lysine best preserved the nanomolar GnRH receptor binding affinity. Further studies are required to con-
firm the existence of these compounds, but a possible way to minimise renal uptake is the co-injection of lysine, which in
the case in In
111
labelled DOTA-
alpha
-melanocyte stimulating hormone peptide caused reductions of up to 70% [55].
7.2.6 n
2
s
2
and n
2
s
4
donor ligands
Gallium and indium complexes containing Ga/In-S bonds are comparatively unusual; the coordination of these metal ions
being dominated by their 'hard' acceptor nature with bonding most commonly found to electronegative non-polarizable
donors such as oxygen and nitrogen. However, Ga(III) and In(III) complexes of the type shown in Figure 7.7 have been pre-
pared and shown to be stable in aqueous solution, where they are cationic with loss of the apical chloride. These ligands were
labelled with
67
Ga,
68
Ga, and unusually, with
113m
In in good yield. All the labelled complexes exhibited high myocardial
uptake
in vivo
in rats but with some washout over time.
Copper
bis
(thiosemicarbazonate) complexes such as Cu(ATSm) have been primarily studied for their hypoxia selectivity,
but this ligand type has recently been used to generate Group 13 complexes of potential interest as a bifunctional chelators
for small peptides and biomolecules. Several straightforward peptide coupling routes are now available with the advent of
bis
(thiosemicarbazone) ligands with pendant carboxylic acid groups (Figure 7.8). However, the
64
Cu-labelled complexes of
R
HN
NH
Cl
M=Ga, In
R=H, cyclohexyl
M
Et
Et
S
S
Et
Et
14
fIGure 7.7
Ga and In complexes of aminothiolate ligands.