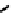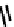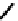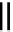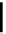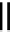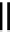Chemistry Reference
In-Depth Information
O
b
3
hAsp-bAla-bAla-BBS-NH
2
NN
N
N
H
M
N
O
SS
H
H
O
15
fIGure 7.8
Structure of a metallic ATSm-Bombesin derivative, m = Cu(II) [57].
N
N
N
N
N
N
N
N
Ga
Ga
S
S
H
H
H
S
S
Cl
Cl
NH
GaClATSM
17
16
fIGure 7.9
Ga complexes of bis(thiosemicarbazones).
these generally suffer from high liver accumulation
in vivo
[56]. Introduction of hydrophilic groups on the ligand exocyclic
structure have lowered the uptake of
64
Cu in the liver somewhat but it still remains high, probably due to the Cu metabolism
liberated from the ligand [56]. In light of this, interest has focused on the synthesis of
67
Ga- and
111
In-radiolabelled
bis
(thiosemicarbazone) ligands to establish if the biodistribution pathways of such gallium or indium chelators was signifi-
cantly different to that of their copper analogues [57].
Whilst it is not expected that the gallium or indium derivatives will be hypoxic selective because they lack metal-based
redox chemistry, the authors' recent work has shown that hypoxic selective groups such as nitroimidazoles can be coupled
to the
bis
(thiosemicarbazone) ligand
via
a hydrazinic linker [58, 59]. Through this strategy hypoxia selectivity may be con-
ferred, albeit indirectly, to the gallium/indium
bis
(thiosemicarbazone) unit, and studies of such complexes will make an
interesting comparison with the known copper analogues.
We recently reported several gallium
bis
(thiosemicarbazonate) complexes (Figure 7.9) [56, 60-62]. and X-ray diffraction
studies showed both symmetric and asymmetric coordination of the ligand, depending on the ligand backbone. An analogous
zinc compound that bound DABCO in the fifth coordination site of the zinc ion has recently been published and shows a
similar asymmetric binding mode (Figure 7.9). Distorted square pyramidal geometries were observed at both gallium(III)
centres in compounds
16
and
17
.
All metal complexes of the
bis
(thiosemicarbazonate) ligand incorporating an aromatic naphthalene backbone (Figure 7.9, e.g.,
compound
17
) studied so far (for m = Zn(II), Cu(II), Ga(III) and In(III)) have been shown to display increased kinetic stability
with respect to their m(ATSm) analogues by virtue of the enforced rigidity of the ligand system. These compounds are intrinsi-
cally fluorescent and show cytotoxicities in the micromolar region in a range of human cancer cell lines [63]. Synthesis of the
gallium and indium complexes is readily achieved by direct
trans
-metallation of the zinc derivative. A similar method was
employed for
68
Ga and
111
In radiolabelling, and this proceeded rapidly in almost quantitative radiochemical yield [60-62, 64].
The cell uptake of the fluorescent indium bis(thiosemicarbazonato) isostructural analogues has been investigated using
confocal fluorescence microscopy [61]. This showed localisation in mitochondria, lysosomes, and additionally for indium
complexes in the nucleus, therefore opening up the possibility of Auger electron emission therapy via
111
In. A recent study led
to the development of a new gallium bis(thiosemicarbazonate) complex for tumour imaging. A
67
Ga-acetylacetonate
bis(thiosemicarbazonate) complex
67
Ga(AATS) was prepared by reaction of
67
Ga acetate with acetylacetonate
bis(thiosemicarbazone) (AATS) for 30 minutes at 90 °C. The radiolabelled Ga complex was prepared with high radiochemical
purity (>97%, HPlC) and shown to be stable in serum. The biodistribution of the labelled compound in wild-type and
fibrosarcoma-bearing rodents was determined and revealed significant tumour accumulation of the tracer at two hours. This