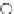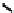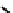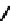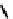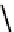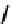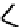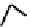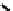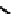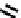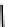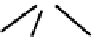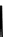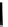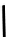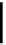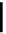Chemistry Reference
In-Depth Information
PR
3
+
+
R
2
P
R
2
P
Cl
N
N
Tc
Tc
R
2
R
2
O
O
Cl
O
O
R=Me, Ph
PR
3
R=CH
2
CH
2
OMe
52
53
fIgure 6.18
Tc(III) complexes with ditertiary phosphine (
52
) Schiff base (
53
) ancillary ligands.
R
B
O
O
O
PMe
2
Ph
N
N
S
N
Tc
S
Tc
S
N
N
S
OBu
X
N
O
O
H
H
O
54
55
fIgure 6.19
Tc(III) complexes with boron-capped dimethylglyoxime (54) and thiolate (55) ligands.
other cationic lipophilic cations such as myoview and Sestamibi share with
51
that uptake in tumours is decreased as Pgp
expression increases, providing a route to assess mDR [187, 188]. Further examples and background appear in a useful
review on mDR imaging [189].
Another well-studied class of Tc(III) complexes are those derived from dimethylglyoxime (dmg) in which three dmg
units are linked together via a boron cap to give a hexadentate n
6
ligand. These are derived from dmg by reaction with
boronic esters and are known as BATo ligands. The complexes formed with Tc(III) are neutral seven coordinate complexes
with a halide X
(54
) completing the coordination sphere [190, 191]. The
99m
Tc analogues can also be made in good yield in
a 'one-pot' reaction from [
99m
Tco
4
]
−
(Figure 6.19).
Despite the neutral charge, this type of complex has been used clinically for heart imaging under the name Cardiotec and
can identify ischemic tissue. However, as with the TcnoET discussed above, the mechanism of uptake and retention of this
neutral complex in myocardial tissue is unknown. However, it does not appear as though the halide is lost
in vivo,
and
replacement of a chloride by hydroxide has little effect on the biological properties [192]. The BATo system can also be used
as a bifunctional chelator; when the boron substituent is 3-isocyanatophenyl, the Tc complex can be linked to monoclonal
antibodies such as B73.3, producing
in vivo
images comparable with those from a radioiodine-labelled analogue [193].
Similarly, derivatisation with a 2-nitroimidazole provided an agent capable of imaging hypoxic tissue [194] (see below for
a more detailed account of hypoxia imaging). Rhenium analogues of BATo ligands have been reported but have not been
radiolabelled [195].
There are many examples of Tc(III) complexes with sulphur ligands; one of the earliest to be investigated and used was
[Tc(tu)
6
]
3+
, which can be used as a precursor for other Tc(III) derivatives. However, the removal of the displaced thiourea can
be troublesome [196]. An X-ray crystal structure showed a pseudo-octahedral coordination with Jahn-Teller distortions
[197]. Reduction of '3 + 1' oxo complexes discussed earlier with tertiary phosphines gives complexes such as
55,
and the
same species can also be prepared directly from [
99m
Tco
4
]
−
in good yields. The geometry about Tc is now trigonal bipyra-
midal rather than square pyramidal, and the absence of the competing π-donating oxo-ligand means that the monodentate
thiolate ligand is a better donor and exchanges more slowly with glutathione than in the oxo species [198, 199]. The trigonal
bipyramidal structure motif is also found for both Tc and Re using sterically hindered thiolate ligands in complexes such as
56
[200] and also for a range of tetradentate 'umbrella' ligands with n or P capping atoms such as
57
[201]. A number of
analogous Re(III) complexes to
56
have also been prepared with axial ligands such as meCn and Co [202] and even n
2
[203]. Although the thiolate ligands in Tc and Re complexes of the type
56
and
57
are likely to be strongly bound, there have
been no radiolabelling or biological studies reported (Figure 6.20).