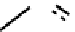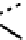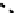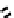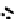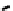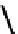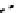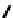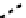Chemistry Reference
In-Depth Information
Me
+
N
Me
Me
Ph
3
P
S
N
Cl
Cl
N
Ph
S
Tc
Re
N
R
1
Cl
S
N
N
Ph
N
Me
Ph
3
P
S
R
2
Me
N
Me
50
51
fIgure 6.17
Isodiazene complexes of Tc and Re.
do not readily convert to diazenides; this has been applied for both Tc and Re. The first Tc hydrazide(2-) complex
[TcCl
3
(nnmePh)(PPh
3
)
2
] was reported in 1990 [171] from the reaction of [TcoCl
4
]
−
with mePhnnH
2
in the presence of
PPh
3
; this was translated to
99m
Tc. The X-ray crystal structure (
50
) was later established [172] for the nnPh
2
derivative and
a range of derivatives prepared via metathesis [173]. The chemistry of Re hydrazides has also been explored, and reaction of
[ReoCl
3
(PPh
3
)
2
] with an excess of mePhnnH
2
gave the cationic five coordinate species [ReCl
2
(nnmePh)
2
(PPh
3
)]
+
.
Subsequent reaction with excess dithiocarbamate gave the very stable six-coordinate complex
51
(Figure 6.17) [174].
Although the hydrazide(2-) ligand is likely to be very inert toward hydrolysis, there have so far been no reports of
in vivo
investigations with this class of ligand.
6.3
technetIum and rhenIum(IV)
The coordination chemistry of the tetravalent oxidation state for both Tc and Re is limited compared to other oxidation states
and generally has not been exploited in a radiopharmaceutical context and will not be discussed here. Comprehensive cov-
erage of the chemistry appears in reviews [15, 16, 28].
6.4
technetIum and rhenIum(III)
The trivalent state for Tc and Re has the electronic configuration d
4
and complexes generally have coordination numbers
from 5 through to 7. The vast majority are diamagnetic, although complexes of the type [ReCl
3
(Pme
2
Ph)
3
] show tempera-
ture-independent paramagnetism and have contact-shifted nmR spectra [175]. As expected for an intermediate oxidation
state, both π acceptor and σ donor ligands bind well, and the coordination chemistry with tertiary phosphines is particularly
extensive. Thus reaction of [
99
Tco
4
]
−
with ditertiary phosphines in DmF gives the octahedral complexes
52,
which can be
extended readily to
99m
Tc. This class of complex showed strong myocardial uptake in canine animal models but, sadly, none
in humans, which was ascribed to reduction of the cationic Tc(III) complex to the neutral Tc(II) derivative
in vivo
[176-178].
Detailed electrochemical studies of the redox properties of both the Tc complexes and their Re analogues have been made
[179]. The complexes could be reduced in two successive reversible one electron processes to Tc(II) and Tc(I) (Figure 6.18).
The complex [TcCl
3
(meCn)(PPh
3
)
2
] can be prepared by the Zn reduction of [TcCl
4
(PPh
3
)
2
] or by reaction of [TcoCl
4
]
−
with meCn and PPh
3
[180]. This is a useful precursor for the synthesis of a range of other Tc(III) complexes such as
[TcCl
3
(py)
3
] (py = pyridine) and [TcCl
2
(bipy)
2
]
+
(bipy = 2,2′-bipyridyl) [181]. The complex [ReCl
3
(meCn)(PPh
3
)
2
] is also
known [182, 183] and was formed from the reaction of [ReoCl
3
(PPh
3
)
2
] with meCn in the presence of PPh
3
. This presents
a similarly versatile intermediate for access to a wide range of rhenium(III) derivatives.
The ability of tertiary phosphines to remove oxo groups from Tc and Re has been exploited in the synthesis of a range of
Tc(III) and Re(III) Schiff base complexes by reactions of mono-oxo complexes with excess of the phosphine. This reaction
is possible for a wide range of tri- and tetra-dentate ligand systems, but the most widely studied in the context of imaging
applications have been complexes of acacen such as
53
. Such complexes could be made in good yield directly from [
99m
Tco
4
]
−
by sequential addition of Schiff base and phosphine and were widely studied as potential myocardial imaging agents. The
furanone and phosphine substituents of
53
were used to optimise the biodistribution characteristics of the compounds [184-
186]. This type of complex was later also found to be potentially useful for the imaging of multidrug resistance (mDR).
Tumour cells can often become resistant to cytotoxins via overexpression of the mDR trans-membrane P-glycoprotein Pgp.