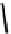Chemistry Reference
In-Depth Information
N
MeCN
Ar
Ar
L=PPh
3.
RNC
S
S
S
S
Tc
S
Tc
Ar
S
L
MeCN
Ar=2,6-Me
2
C
6
H
3
56
57
fIgure 6.20
Thiolato Tc(III) complexes.
6.5
technetIum and rhenIum(I)
6.5.1
Isocyanide complexes
In the 1960s, the early days of the development of technetium chemistry, it would have been regarded as fanciful to suggest
that organometallic complexes might be used as radiopharmaceuticals. Syntheses were usually conducted in dry organic
solvents in an inert atmosphere; conditions far removed from those used in a radiopharmacy. The first hints that Tc-C bonds
might be viable for biological applications came with the synthesis by Davison, Jones et al. of extremely stable, water-soluble
isocyanide complexes of the type [Tc(CnBu
t
)
6
]
+
in 1982 [204]. The initial synthesis was from the Tc(III) hexakis(thiourea)
complex, but it was later shown that the same class of complex could be made directly from [
99m
Tco
4
]
−
using dithionite as reduc-
tant and a large excess of the isocyanide ligand. The same paper also showed that the complex could be used as a myocardial
imaging agent and that it performed as well as
201
Tl, which was used clinically at that time [205, 206]. The biodistribution
characteristics were later optimised by using a methoxybutyl isocyanide substituent TcmIBI, which entered clinical use
under the trade name Cardiolite. The mechanism of uptake in myocardial tissue involves passive diffusion into cells followed
by trapping in mitochondrial membranes due the negative potential across the membrane [207, 208]. Cancer cells have
increased metabolism, resulting in a more negative potential; TcmIBI can therefore also be used for imaging tumours [209].
Analogous rhenium hexakis(isocyanide) cations can be prepared by reaction of [ReoCl
3
(PPh
3
)
2
] [210] or complexes with
Re-Re multiple bonds [211] with an excess of isocyanide, but no radiolabelling or biological studies have been reported.
6.5.2
tricarbonyl complexes
Some purists might claim that isocyanide complexes, while containing a m-C bond, are not truly organometallic complexes.
However, there is no doubt that the [m(Co)
3
Cl
3
]
2−
species first reported by Alberto et al. in 1995 [212] by the low pressure
carbonylation of [Tco
4
]
−
in the presence of reductant is organometallic. The imaging potential of this species and the slightly
later reported [Tc(Co)
3
(H
2
o)
3
]
+
[213] was quickly realised, and this class of compound has become one of the most used
over the last 20 years for the development of new
99m
Tc-based diagnostic agents, thus representing a significant break-
through. There have been far too many publications covering applications of this class of complex for a comprehensive
account here, so we therefore present only a summary of the underlying chemistry and some selected examples. There
have been several detailed reviews by Alberto et al. that give much other useful information [214-217]. The main focus of
research on Re tricarbonyl complexes has been on their use a cold surrogates for the technetium analogues. Although the
tri-aquo Tc cation could be made from [Tco
4
]
−
, the use of Co gas was not appropriate for radiopharmaceutical applications.
A crucial step was the discovery that boranecarbonate ([H
3
BCo
2
H]
−
) could be used with [
99m
Tco
4
]
−
in water as a simulta-
neous reductant and Co source, giving a high yield of the tricarbonyl derivative [218]. This enabled a kit formulation to be
developed that is now available commercially under the name Isolink
Tm
. The Re analogue can also be made from [
188
Reo
4
]
−
by modifying the conditions to include H
3
B.nH
3
as well as the boranecarbonate [219, 220]. Several factors contribute to the
especial suitability of the tricarbonyl core for radiopharmaceutical applications. Complexes of the type [m(Co)
3
l
3
]
+
(where
l is a neutral ligand) are octahedral with a d
6
electron configuration, which confers kinetic stability of the complexes due to
loss of crystal field stabilisation energy going to five or seven coordinate transition states. The Co ligands are strongly bound
due to their π-acceptor properties and the low valent electron rich m(I) ion. moreover, the balance of electron density bet-
ween metal and ligand is such that the Co ligands are not open to degradation via nucleophilic or electrophilic attack at the
Co carbon. The competition between the Co ligands for metal electron density leads to their obligately facial arrangement
at the metal centre, which confers stereochemical rigidity on the complexes. The use of a polydentate ligand at the other
three sites prevents any ligand exchange reactions. These complexes, like many classic organometallics, also conform to
the 18-electron rule. The strong π-bonding creates a wide separation between the stabilised Homo bonding and lUmo