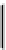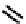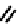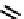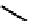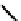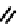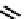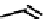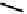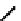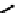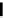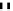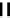Chemistry Reference
In-Depth Information
HO
O
O
O
O
OH
N
N
O
N
N
O
Tc
Tc
N
N
S
O
S
O
COOH
COOH
OH
18
19
OH
O
O
S
S
CO
2
H
O
HO
2
C
N
N
O
HN
M
Tc
CO
2
H
HO
2
C
S
S
N
S
O
HOOC
M=Tc, Re
20
21
fIgure 6.6
Aminoacid based and DmSA complexes.
the monoamide ligand theme is the replacement of the neutral amine donor by thioether. The
99
Tc and Re(V) oxo complexes
of a series of such ligands (
16
) have been reported, and variations of backbone lengths and substitution patterns were shown
to have little impact on stability or ease of synthesis. The X-ray structures of three Re(V) oxo-complexes were reported [65].
Another n
2
S
2
ligand system that has been investigated with technetium features bis(thiosemicarbazones) such as ATSm
(
17)
and a variant with a single methyl group on the backbone (KTS)
.
Reaction of [
99m
Tco
4
]
−
with
17
in the presence of stan-
nous chloride gives a very stable complex that has not as yet been fully characterised. It has been suggested [66, 67] that it
is a neutral Tc(IV) oxo-complex, but a Tc(V) complex with an anionic ligand such as halide
trans
to the oxo-group would
seem more likely. An analogous
99m
Tc complex derived from a bis(thiosemicarbazone) of glucose has also been prepared
with
99m
Tc. Interestingly, in animal studies this crosses the blood brain barrier suggesting possible applications for brain
imaging [68].
Amino acids are an obvious source of amide donors, and Tc and Re complexes of both peptides and ligands containing
peptide sequences have been widely pursued. The first to be described in detail by Fritzberg et al [69, 70]. was mAG
3
(mercaptoacetylcysteinetriglycine) (
18
), which has found widespread use as a kidney imaging agent (TechneScan) (Figure 6.6).
This complex has also been widely used for the
99m
Tc labelling of biomolecules, and examples appear in the reviews quoted
above. The potential issue of structural isomers has been discussed in a review covering Tc radiopharmaceuticals [71]. The
X-ray structure of ReomAG
3
has been reported [72], and it was shown that pre-conjugation of somastatin analogue peptides
was preferable when labelling with
188
Re [73]. Biodistribution studies in mice showed high tumour uptake for the conjugate
with values comparable to those achieved by direct labelling [74]. A similar approach has been used to radiolabel monoclonal
antibodies with
188
Re for RAIT (radioactive immune therapy) [75]. A comparison of labelling moRFs (synthetic DnA
equivalents) using
188
RemAG
3
and
90
y-DoTA showed some loss of both radionuclides although both were virtually intact
after 48 h [76, 77]. other examples of radiorhenium labelling of biomolecules using mAG
3
analogues appear in a review of
bifunctional chelators for Re [6].
It was later reported that the Tc complex of the triserine analogue mAS
3
(
19
) was a useful alternative to mAG
3
for both
renal imaging and protein labelling [78]. A comparative study of the Tc labelling of neutrophil elastase inhibitor protein
(HnE-2) in primates using Tc-mAG
3
, TcmSER
3,
Tc-DTPA, and Tc-HynIC was carried out [79]. In terms of SPECT
imaging, all of the compounds showed similar levels of accumulation in the lesion, although there were differences in the
distributions in the major organs. This accentuates the problem of selecting an optimal bifunctional chelator for a given
protein because, although based on very distinct Tc coordination chemistries, the actual differences in targeting ability may
be relatively incremental.
An alternative to the bifunctional chelator/conjugation approach is to engineer a Tc binding site within a protein sequence.
The use of the Cys-Gly-Cys, Gly-Gly-Cys, and lys-Gly-Cys motifs for binding
99m
Tc in a peptide system for targeting
somastatin receptors has been reported, and the binding of the Tc did not compromise receptor binding. In fact, the affinities
of some of the Re(V) analogues were higher than the free proteins. The
99m
Tc labelled complexes showed good tumour reten-
tion in rats [80]. Similar Tc and Re binding sequences were used to label a protein to target the GPIIb/IIIa receptor as a
means of thrombus imaging, and again metal binding did not interfere with biological activity [81]. The RP414 ligand