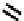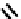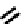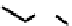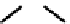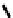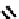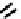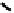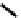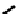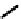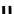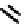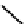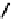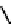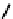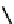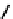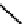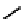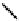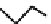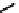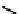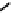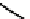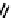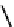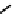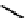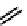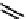Chemistry Reference
In-Depth Information
system (
20
) for binding Tc or Re contains the dimethylglycine-serine-cysteine-glycine sequence and the
99m
Tc labelled form
has been successfully conjugated to neurotensin for tumour imaging
in vivo
[82]. An interesting solid phase synthetic
approach involving the initial binding of
20
to a gold surface via the cysteinyl sulphur has been reported [83]. on binding
Tc, the gold-sulphur bond cleaves and the Tc complex moves into solution, leaving the excess ligand on the surface, thereby
increasing the specific activity of the product.
A great advantage of utilising a peptide binding motif for the metal is that it can readily be fine-tuned to optimise
in vivo
distribution. An illustrative example is provided by the Tc Depreotide system (
22,
also known as neoTect) for the imaging
of somastatin receptors, which are overexpressed in a range of cancer types (Figure 6.7). It comprises a variant of the cyclic
octreotide and a Dap-lys-Cys (Dap = 2,3 diaminopropionic acid, a non-naturally occurring amino acid) motif to bind the
Tco
3+
core. This compound has been clinically approved and is in regular use for imaging of pulmonary masses [84, 85].
However, when it comes to using the same system for radiotherapy using
188
Re, the biodistribution creates significant prob-
lems.
In vivo
Tc-Depreotide shows high retention in the kidneys, which would be a significant problem for the
188
Re species
because it would result in unacceptably high doses to a major internal organ. Also the tumour-to-muscle ratio for the retained
radionuclide is somewhat on the low side. Through some elegant peptide synthesis both problems were addressed.
modification of the cyclic peptide by interchanging cysteine and phenylalanine and substituting threonine for valine
improved the IC
50
value from 1.5 nm to 0.1 nm. Also, modification of the metal binding motif to that shown in
23
reduced
the kidney uptake from 152% ID/g to 5% ID/g. The Re compound
23
is now in clinical trials, and the above illustrates the
much more stringent measures that need to be taken when using a radionuclide for therapy.
The chemistry of Re and Tc with dithiolate ligands can be complex, but the DmSA ligand forms well defined and very
stable complexes (
21
) with both elements [86]. The Tc complex has found some use for imaging kidney function and is
remarkably stable
in vivo,
being excreted essentially intact [87]. The Re complex can be converted readily into a dianhy-
dride, which was then conjugated under mild conditions to two molecules of salmon calcitonin. Acetylhydrazine can be used
as a reductant for the Re; it has the advantage of giving rise to a single isomer [88].
A somewhat different strategy has been used to achieve targeting of rhenium and technetium complexes using a
combination of tri- and mono-dentate ligands - the so called '3 + 1' approach (Figure 6.8). The Re(V) complex
24
is designed
to target dopamine transporter sites and contains an α-tropanol derivative linked via a monodentate thiol, and the complex
NH
2
NH
2
NH
2
HO
O
O
PheNMe Tyr D-Trp
O
O
N
O
O
O
O
N
N
N
N
O
H
2
N
H
CysH Val Lys
NMe
Cys
Tyr D-Trp
M
O
N
M
NH
2
N
OH
S
H
2
S
N
H
2
O
Phe Thr Lys
O
22
23
fIgure 6.7
Peptide-based ligands for Tc and Re.
NMe
O
O
S
S
S
O
O
S
N
O
O
Re
Tc
S
S
S
S
24
25
O
S
Cl
O
NH
N
O
Re
Re
S
S
O
S
N
S
N
NO
2
S
O
26
27
fIgure 6.8
Examples of the '3 + 1' approach.