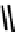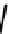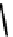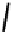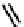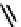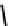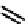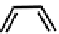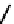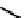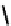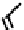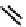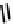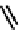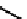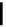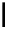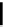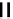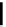Chemistry Reference
In-Depth Information
HO
OH
NH
N
NH
N
HN
N
HN
N
O
OH
HO
O
6
7
3-
OCH
2
COOH
OCH
2
COOH
SO
3
H
O
NH
N
N
O
N
O
O
SO
3
H
HOOCH
2
CO
O
Re
HO
3
S
Tc
N
HN
N
N
O
O
HOOCH
2
CO
O
OCH
2
COOH
OCH
2
COOH
SO
3
H
8
9
fIgure 6.3
Porphyrin ligands and complexes.
oxo group, the complexes are quite labile. Even weak donors such as ethylene glycol are able to bind, and two carboxylate
oxygens are displaced from the coordination sphere [47]. The structure of the Tc complex formed with DTPA is not certain,
although it has been suggested to be a mono-oxo species with the DTPA ligand bound only via carboxyl oxygens. It has been
widely used for kidney imaging where it indicates glomular filtration rates. It has also been used for the labelling of biomol-
ecules. Several reviews have appeared covering different features of the radiolabelling of biomolecules with technetium and
rhenium [17, 31, 48-51].
6.2.1.3 N
x
S
4-x
Donor Ligands
This classification encompasses the largest group of ligands used in conjunction with the
Tc(V) and Re(V) oxo cores. many variations have been reported and all show high serum stability. This section contains
selected examples that have been tested
in vivo
or show interesting features of their coordination chemistry. one prevalent
type is the diammine dithiolates variously designated as DADT or BAT ligands. A variety of backbone lengths have been
explored, but the ethylene variant has been the most common choice. Figure 6.4 shows some variants on the theme.
methylation of one of the backbone nitrogens in
10
ensures that a neutral complex is formed on reaction with pertechnetate.
In common with many other oxo-complexes of tetradentate ligands, the monosubstituted derivatives or conjugates lack C
2
symmmetry and
syn
or
anti
isomers with respect to the oxo group are possible. Conjugation to biomolecules can be achieved