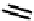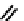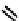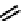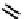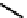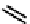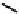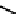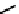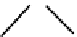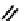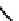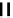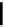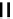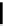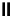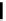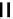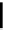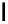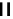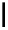Chemistry Reference
In-Depth Information
O
N
O
N
N
N
Tc
Tc
N
N
N
N
O
O
O
O
H
H
1
2
fIgure 6.1
Amineoxime complexes.
-
O
O
O
O
O
O
O
O
O
N
N
N
N
N
N
M
Tc
Tc
X
N
N
N
N
N
O
H
2
O
O
M=Tc, X=Cl
M=Re, X=OEt
CO
2
H
4
3
5
fIgure 6.2
Amineamido and amido ligands.
However, a related diammidopyridyl ligand forms both Tc and Re water-soluble mono-oxo complexes
5
and X-ray struc-
tures of both were described, but radiolabelling was not reported [38].
Porphyrins are potentially attractive ligands for technetium and rhenium because the complexes are extremely robust and
also fluorescent. Generally, the insertion of the metals requires fairly forcing conditions such as dichlorobenzene under
reflux [39]. However, it has been reported that a Tc(V) mono-oxo complex of octaethylporphyrin
6
can be prepared from
pertechnetate by heating under reflux in glacial acetic acid, but radiolabelling and biodistribution were not reported
(Figure 6.3) [40].
Haematoporphyrin
7
was radiolabelled with
99m
Tc, which was shown to bind at the carboxyl groups [41, 42]. Strong reten-
tion in adenocarcinomas was observed
in vivo.
This nonspecific uptake appears to be a common feature of metalloporphyrins
and is exploited in photodynamic tumour therapy. The carboxyl-substituted tetraphenylporphyrin
8
has been labelled with
99m
Tc at the peripheral carboxylates, and the compound was shown to accumulate in a variety of murine tumours [43].
This binding to the exocyclic donors shows that for any porphyrin-bearing potential donor groups, metallation within the
porphyrin ring cannot be assumed. An analogous porphyrin with pendant cyclam groups has also been labelled with
99m
Tc
[44, 45]. It has been claimed that the sulphonated porphyrin
9
forms a rhenium(V) oxo-complex directly from perrhenate in
water at 100°C with stannous tartrate as reductant. However, the structure was not verified, and some form of coordination
to the sulphonate groups cannot be excluded.
The strong nonspecific binding of porphyrins is an issue when using small biologically active molecules as targeting
vectors, and conjugation to larger groups such as antibody fragments would appear to be required to achieve specific targeting.
The size of the targeting group will need to be selected to ensure the pharmacokinetics are not too slow for the 6 hr half-life
of
99m
Tc. This approach does not appear to have been explored as yet for technetium but has been with other radioisotopes.
6.2.1.2 N
x
O
4-x
Donor Ligands
The major types of ligand in this category that have been explored have been Schiff bases.
There are reported examples of both Tc and Re mono-oxo complexes with well-known ligands such as salen and salphen,
but these have not been used in a radiopharmaceutical context and will not be discussed further. Polyaminocarboxylates such
as DTPA, DoTA and noTA have been used extensively with other radionuclides as bifunctional chelators, but their behaviour
with technetium and rhenium is not straightforward. Reaction of [TcoCl
4
]
−
with H
4
EDTA in anhydrous DmF gives an
unusual seven coordinate Tc(V) oxo-complex [Tco(EDTA)]
2−
isolated as a barium salt [46]. The two nitrogens are coordi-
nated approximately
trans
to the oxo-group with four carboxylates in an equatorial plane. With the high
trans
effect of the