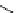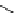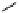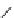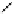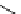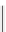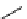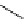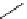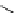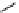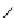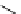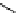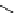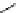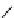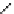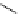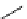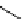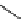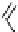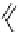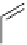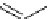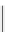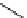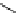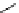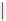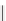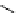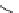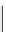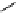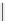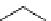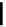Chemistry Reference
In-Depth Information
HO
2
C
CO
2
H
CO
2
H
NH
HN
N
N
N
N
N
N
N
N
NH
HN
HO
2
C
CO
2
H
HO
2
C
CB-TE2A
Cyclam
TETA
O
O
N
N
N
N
NH
NH
2
N
N
N
N
HO
2
C
HO
2
C
CB-TEAMA
CB-PhTEAMA
fIgure 5.13
The macrocycle cyclam, as well as its derivatives TETA, CB-TE2A, CB-TEAMA, and CB-PhTEAMA.
coordination geometry, complex stability, and often decomplexation [143]. The acid inertness and
in vivo
stability of copper
complexes has been successfully compared to their reduction potentials [35, 143]. The cyclam- and cyclen-based macro-
cycles have demonstrated that higher reduction potentials resulted in less stable complexes, with the most stable copper
chelator CB-TE2A having the lowest reduction potential as well as a
quasi
-reversible Cu(II)/Cu(I) reduction [35, 143].
When radioactive copper complexes dissociate
in vivo
, the radiometal can be observed to accumulate in the liver, most
likely associating with the proteins ceruloplasmin and/or superoxide dismutase [78, 144]. The association of free radiocop-
per with ceruloplasmin and superoxide dismutase in the liver has been demonstrated by the
in vivo
demetallation of an
unstable
67
Cu-TETA-antibody conjugate [144].
human serum albumin (hsA) is the most abundant serum protein in human blood, and its biological function is the non-
specific binding of various metal ions, drugs, and other small molecules [118]. hsA contains four metal ion binding sites,
with site 1 being of particular interest for
64
Cu-based radiopharmaceuticals, because it strongly binds copper(II) and nickel(II)
[145]. Although only hsA binding site 1 is known to specifically bind any of the radiometals discussed in this chapter, it can
still accommodate other metal ions to varying degrees and may exhibit nonspecific binding to radiometals other than
64
Cu
[145].
5.6.4
64
copper radiometallation protocols
Because
64
Cu has been so hotly investigated, there exists a large variety of labelling conditions with many different chelators.
nOTA and dOTA conjugates are typically radiolabelled between ph 5-8 with temperatures ranging between 40-80 °C [140,
146-148], and CB-TE2A conjugates typically require high temperatures and long reaction times (60 minutes at 90 °C) [141,
149]. The chelators diamsar and TETA can be radiolabelled under mild ambient temperatures in ~60 minutes and are there-
fore more compatible with antibody biovectors [149, 150]. Many
64
Cu antibody conjugates (targetting hER2, CEA, EGFR,
PsMA, Gd2, CC, etc.) have been radiolabelled, typically in 15-60 minutes and at 25-40 °C, depending on the chelator
[151-153]. Many other peptides and antibodies have been conjugated to these chelators and radiolabelled with
64
Cu; only a
few representative examples have been discussed.
5.6.5
64
copper summary
64
Cu is intrinsically a dual-modality radiometal with both β
-
emission for therapy and β
+
for PET imaging, negating the need
for a matched isotope pair. The intermediate half-life of 12.7 hours allows it to be used with a wide variety of biovectors
(peptides, antibodies, etc.); however, it may not be long enough to optimally match the slow 1- to 3-day localisation times
of fully intact antibodies (~150 kda) and image 3 to 7 days post-injection. despite this possible limitation, many antibody
conjugates have been successfully imaged 24-48 hours after using
64
Cu without issue.
64
Cu has ideal decay properties with a
low-energy β
+
emission that provides high-resolution PET images and low energy γ emissions that subject patients to low
radiation burdens. The natural lability of Cu(II) complexes, the relatively facile redox chemistry between Cu(II)/Cu(I), and
the abundance of
in vivo
competition from native copper-rich proteins means that stability problems often arise, and design
of thermodynamically stable and kinetically inert chelators is a significant challenge.