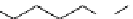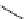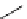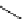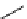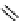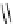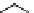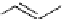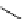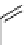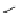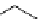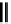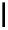Chemistry Reference
In-Depth Information
5.7
89
ZIrconIum radIometal Ion propertIes
89
Zr has recently become very popular in the literature, and the last five years have seen a drastic increase in efforts to trans-
late
89
Zr-based imaging agents to the clinic [22]. Zr(IV) is a highly charged and extremely hard metal ion with a relatively
small ionic radius (84 and 89 pm for Cn = 8 and 9, respectively [15]), and an exceptionally low p
K
a of 0.22 (Table 5.2). The
hard nature of Zr(IV) shows in its preference for hard carboxylate and hydroxamate-oxygen anions.
89
Zr has the longest
half-life (78.5 hours) of the radiometal PET nuclides discussed in this chapter and is ideally suited to theranostic applications
as a matched isotope pair for long-lived therapeutic isotopes such as
90
Y and
177
lu.
90
Y and
177
lu have long half-lives (64.1
and 161 hours, respectively); to provide accurate imaging/dosimetry data at time points of 1 to 7 days post injection there
must be an appropriate surrogate radiometal (ideally a positron emitter for PET) with established chemistry and chelate sys-
tems. long-lived isotopes such as
89
Zr are ideally matched with antibody biovectors, because they require 2 to 3 days to fully
localise and penetrate tumours (although imaging can be effectively performed after 24 hours) [26].
89
Zr is the least investi-
gated isotope of those presented in this chapter; however, it has been investigated as a longer half-life alternative PET isotope
to
68
Ga for BFC-based radiopharmaceuticals.
89
Zr is thought to be superior to
111
In for dosimetry because it is a β
+
emitter
and provides more accurate and quantitative biodistribution data. The low energy β
+
emission of
89
Zr (897 keV) provides
high-resolution PET images; however, emission of high-energy γ rays in combination with its long half-life significantly
increases the absorbed dose that patients receive. One of the major advantages of
89
Zr over other PET radiometals is that it
is retained in cells after being internalised, which could provide essentially irreversible cellular delivery [154-156].
89
Zr is
typically cyclotron-produced via the nuclear reaction
89
Y(p,n)
89
Zr, and purified by anion exchange chromatography by
elution from a solid-phase hydroxamate resin with 1 M oxalic acid [157].
5.7.1
clinical trials Based on
89
Zirconium
no FdA-approved radiopharmaceuticals currently utilise
89
Zr. due to the long half-life of
89
Zr (78.5 hours) it is perfectly
matched with the biological half-life of antibody biovectors, which can circulate
in vivo
for weeks [26]. nearly all BFC work
performed with
89
Zr relies on derivatives of the bacterial siderophore desferrioxamine (dFO) (Figure 5.14), [158], which
binds
89
Zr with its three hydroxamate groups in a hexadentate fashion [159].
dTPA has been shown to form a very thermodynamically stable complex with Zr(IV) (log
K
Ml
= 35.8-36.9); however,
inferior
in vivo
stability has limited its use [160]. Although dFO has been the 'gold standard' for Zr(IV) chelation, its solu-
bility is poor and causes significant synthetic challenges.
89
Zr-dFO-Zevalin was the first
89
Zr antibody conjugate imaged in
humans and was shown to be a suitable PET surrogate for
90
Y-Zevalin dosimetry [161].
89
Zr-dFO-u36 (anti-Cd446 chimeric
mAb) was in clinical trials for squamous cell carcinoma imaging and gave comparable diagnostic results as
18
F-FdG [53,
162]. Another similar example is
89
Zr-dFO-bevacizumab, where bevacizumab is an antibody that targets vascular endothelial
growth factor (VEGF), which is a soluble ligand for the VEGF-receptor that is over-expressed in many cancers and that reg-
ulate angiogenesis [53, 163]. Antibodies such as bevacizumab that bind to VEGF provide a way to both inhibit angiogenesis
in tumours and provide imaging or therapy if an appropriate radionuclide is conjugated to the antibody [53, 163].
89
Zr-dFO-
bevacizumab has demonstrated clear and significant tumour localisation after 72 hours [53, 163].
89
Zr-dFO-trastuzumab is
in clinical trials for imaging breast cancer and
89
Zr-dFO-cetuximab for imaging head, neck, and colorectal cancer [53, 164].
O
O
DFO
NCS
N
S
O
O
DFO-Chx-Mal
H
DFO
O
HN
DFO-
p
-SCN
N
HO
5
HO
N
5
O
R
HN
O
H
N
O
O
OH
X
X
= I, Br
DFO
Desferrioxamine (DFO)
DFO-IAC, DFO-BAC
fIgure 5.14
The most commonly used
89
Zr chelator, desferrioxamine (dFO), with various BFC precursors.