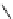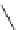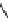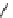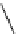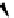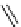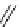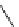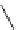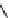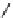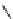Chemistry Reference
In-Depth Information
H
H
H
H
N
N
N
N
R′
R
NH HN
NH HN
H
2
N
NH
2
HN
NH
N
N
N
N
H
H
H
H
Diamsar
SarAr
R
=NH
2
,
R′
=H
AmBaSar
R
= COOH,
R′
=H
BaBaSar
R
= COOH,
R′
= -CH
2
-Ph-COOH
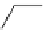
fIgure 5.12
The copper chelator diamsar and three of its BFC precursors sarAr, AmBasar, and BaBasar.
level of oxygen depletion in tumours [53, 128-132]. The emission of β
-
particles from
64
Cu renders
64
Cu-ATsM a radiothera-
peutic as well as an imaging agent. The combined β
+
and β
-
emission of
64
Cu allows for imaging/dosimetry and therapy from
the same isotope, alleviating the need for a second nuclide to be used as a matched isotope pair.
The chelator diamsar was reported to quantitatively radiolabel
64
Cu much faster than other macrocyclic chelates and
was even able to radiolabel at room temperature (Figure 5.12) [133, 134]. The bifunctional derivative of diamsar, sarAr,
was successfully conjugated to the prostate cancer-targeting peptide bombesin, as well as the whole and fragmented
antibody B72.3, whilst retaining its room temperature labelling abilities [133, 134]. A bivalent (two biovectors attached
to one BFC) diamsar-based BFC, BaBasar, has recently been reported and conjugated to RGd [135]. BaBasar-RGd
2
was quantitatively radiometallated with
64
Cu in 5 minutes at room temperature and demonstrated high stability as well as
improved tumour targeting over monovalent AmBasar-RGd conjugates [135]. The BFCs nOdAGA, CB-TE2A, and
dOTA were conjugated to the same novel somatostatin receptor antagonist
p
-Cl-Phecyclo(d-Cys-Tyr-d-4-amino-
Phe(carbamoyl)-lys-Thr-Cys)d-Tyrnh
2
(lM3) and radiolabelled with
64
Cu and
68
Ga [136]. The results demonstrated
that
64
Cu/
68
Ga-nOdAGA (Figure 5.9) had superior tumour-to-normal-tissue ratios and better clearance of non-receptor-
bound BFC from the blood pool, when compared to the
64
Cu/
68
Ga complexes of CB-TE2A and dOTA [136]. Receptor
binding affinity was significantly modulated by up to 10-fold depending on the chelator and metal used, even when
attached to the same targeting vector (lM3), demonstrating the strong affect that chelates can have on radiopharmaceu-
tical pharmacokinetics [136].
5.6.2
recent
64
copper work
Of all the investigated copper chelators, the cylcam/TETA-based chelator CB-TE2A appears to be one of the most promising
(Figure 5.13) [35]. CB-TE2A shows high stability with Cu(II) and impressive kinetic inertness as demonstrated by acid
decomplexation studies performed in 5 M hCl [35]. Animal studies performed in mice demonstrated that the mono-amide
functionalised derivatives of CB-TE2A,
64
Cu-CB-TEAMA, and
64
Cu-CB-PhTEAMA had comparable stability to CB-TE2A
and were suitable candidates for further elaboration as BFCs (Figure 5.13) [58]. Although CB-TE2A and its derivatives
appear to be the best radiocopper chelating agents, they typically require radiolabelling conditions of ~70-95 °C, which,
although suitable for peptide biovectors, is not ideal for sensitive biomolecules such as antibodies [58, 59]. The recently
published CB-TE2A derivatives CB-TE2P and CB-TE1A1P contain phosphonate pendant groups. They have been shown to
radiolabel
64
Cu at ambient temperature in high specific activities and show comparable
in vivo
stability to CB-TE2A [137].
Although dOTA has been used most extensively as a BFC agent for
64
Cu with both peptides and antibodies, it has been
shown that it does not form an optimally stable complex with
64
Cu, as observed
in vivo
by high liver uptake indicating release
of free
64
Cu (
vida infra
) [138, 139].
64
Cu-nOTA-RGd (
cyclic
-Arg-Gly-Asp, bioactive peptide that targets integrin α
v
β
3
expression) and
64
Cu-nOTA-BBn (bombesin, peptide that targets the gastrin-releasing peptide receptor) bioconjugates have
been synthesised from
p
-sCn-Bn-nOTA (Figure 5.9). Although radiometallation was fast (15 minutes, 40 °C), high liver
uptake was observed, suggesting poor
in vivo
stability [140]. A
64
Cu-nOdAGA-
c
(RGdfK) conjugate has also been recently
reported and radiolabelled in high specific activity that demonstrated very promising stability and tumour uptake, similar to
that of
64
Cu-CB-TE2A-
c
(RGdfK) [141].
5.6.3
stability of
64
copper-Based radiopharmaceuticals
Copper is very important for living organisms, and its homeostasis is tightly regulated in biological systems by a multitude
of transport proteins and enzymes, which all serve as
in vivo
competition for BFC coordination [142]. Copper can also be
redox active
in vivo
, and Cu(II) may be reduced to Cu(I) via ascorbic acid, which would result in a change in chelate