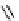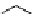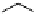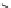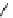Chemistry Reference
In-Depth Information
5.5.5
68
gallium summary
68
Ga has well-developed coordination chemistry and very attractive nuclear decay properties (high positron abundance),
making it ideal for incorporation into BFC-based radiopharmaceuticals for PET imaging. The short half-life of 68 minutes
makes
68
Ga ideal for imaging with rapidly localising and clearing biovectors, such as small peptides (i.e., RGd, octreotide,
bombesin, antibody fragments), while minimising patient-absorbed doses. The commercialisation and availability of efficient
68
Ga generators have allowed research efforts to expand quickly and have made it one of the most promising exotic metal
nuclides for PET imaging. Because Ga(III) has very similar properties to Fe(III), the iron transport protein transferrin presents
a significant stability challenge
in vivo
and requires the use of very thermodynamically stable and kinetically inert chelators.
5.6
64
copper radIometal Ion propertIes
Radiocopper is typically used in its 2+ oxidation state for radiopharmaceuticals, although oxidation states of +1/+2/+3 are
accessible in aqueous conditions [18, 25]. Cu(II) is a first row transition metal with a d
9
configuration and a preference for bor-
derline soft ligand donor atoms such as amines, imines, and thiols [18, 25]. Cu(I) (d
10
ion) prefers softer donor atoms, and its
complexes are less utilised because they are quite labile and lack the required kinetic inertness to withstand physiological con-
ditions [18, 25]. Copper forms tetra-, penta-, and hexa-dentate complexes, typically with square planar, trigonal bipyramidal,
square pyramidal, and distorted octahedral geometries (Jahn-Teller distortions) [18, 25]. By using hexadentate chelators, the
coordination sphere of Cu(II) can be saturated in a distorted octahedral geometry to minimise interactions with exogenous
ligands and biological chelators and maximise stability and kinetic inertness [18, 25]. due to the fast reaction kinetics of Cu(II)
(as demonstrated by its fast water exchange rate of 2 × 10
8
s
-1
, Table 5.2), complexes must be made that are very inert [16, 25].
unlike Ga(III) and Y(III), Cu(II) can be complexed/radiometallated below ph 7 without insoluble hydroxide formation,
because it has a lower affinity for hydroxide ions. The high lability of Cu(II) complexes makes their successful incorporation
into radiopharmaceuticals more difficult than Ga(III), Y(III), and Zr(IV) [25].
64
Cu is a unique metal nuclide because it has
a moderate half-life of 12.7 hours and emits both β
+
and β
-
particles, allowing it to be used as a multipurpose PET/therapy
nuclide. In contrast to
86
Y and
89
Zr,
64
Cu has much lower energy γ emissions, which expose patients to lower radiation bur-
dens.
64
Cu has a lower energy β
+
emission than does
68
Ga or
86
Y, providing higher resolution images.
64
Cu is typically
cyclotron-produced via the nuclear reaction
64
ni(p,n)
64
Cu and purified by anion exchange (i.e., AG1-X8) [126, 127]. A
number of other isotopes of copper emit positrons:
60
Cu (t
1/2
= 23.7 minutes),
61
Cu (t
1/2
= 3.3 hours), and
62
Cu (t
1/2
= 9.7 min-
utes); however, these isotopes are not as heavily investigated due to their shorter half-lives and higher positron energies.
62
Cu
is a
62
Zn generator-produced copper isotope with a very short half-life of 9.7 minutes. Although
62
Cu is useful for experi-
ments that require imaging at very short time points and for minimising radiation dose to patients,
62
Cu has not generated as
much interest as the longer lived isotope
64
Cu.
62
Cu-PTsM has garnered significant interest for imaging the heart and tumour
hypoxia; however, the short half-life means the generator system only lasts 1-2 days and therefore presents a significant
logistical problem requiring frequent delivery and an overall high cost of operation (Figure 5.11) [128-132]. Another short-
coming of
62
Cu-PTsM is poor
in vivo
stability, resulting in high liver uptake of free
62
Cu [128].
5.6.1
clinical trials Based on
64
copper
64
Cu is not incorporated into any FdA-approved radiopharmaceuticals, and its current clinical trial offerings [53] use BFC
systems similar to those used for the previously discussed metal nuclides. The dOTA antibody conjugate
64
Cu-dOTA-
trastuzumab is currently in clinical trials for imaging and therapy of breast cancer [53, 98]. The
64
Cu-ATsM chelate is a more
unique entry, because the chelator ATsM is not functionalised for conjugation to a biovector (Figure 5.11) [53, 128-132].
The
64
Cu-ATsM complex has high affinity for hypoxic tumour tissue and can be used as a way to image and measure the
H
3
C
R
N
N
N
N
CH
3
H
3
C
H
SH
HS
H
PTSM,
R
=H
ATSM,
R
=CH
3
fIgure 5.11
The copper ligands PTsM and ATsM, used for imaging the heart as well as tumour hypoxia.