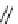Chemistry Reference
In-Depth Information
Radiometal BFC-
complex conjugated
to a targeting
biovector
Target receptor
saturation by
non-radiolabelled
BFC-conjugate
Demetallation
in vivo
Bone
uptake /
adsorption
Excretion
(urine,
feces)
Metal containing
enzymes
(ceruloplasmin,
superoxide dismutase)
Metal storage
(ferritin)
Metal transport proteins
(transferrin, lactoferrin,
metallothionein)
fIgure 5.3
some of the possible biological fates and consequences of BFC radiodemetallation
in vivo
(solid-state structures of ferritin
h-chain homopolymer PdB file 1FhA, ceruloplasmin PdB file 2J5W, and
apo
-transferrin PdB file 2hAV shown).
demetallated radiopharmaceutical in circulation (now non-radioactive), the target receptors can become saturated and then
block any further binding by the circulating population that is still radioactive [23]. The radiopharmaceutical must therefore
have a high apparent specific activity (high quantity of radioactivity per mass unit of compound) and be remarkably stable
and inert. small molecule agents containing covalently bound radiohalogens such as
124
I are usually challenged by enzymatic
degradation, and once metabolised, the radioiodine is either excreted/accumulated as a metabolite of the parent radiophar-
maceutical or as free radioiodide (which typically localises in the thyroid and stomach).
5.4
86
yttrIum radIometal Ion propertIes
Yttrium is the largest of the metals discussed in this chapter and typically forms eight or nine coordinate chelate complexes,
with the most common geometries being square antiprismatic and monocapped square antiprismatic. Y(III) is the least acidic
metal ion discussed in this section (p
K
a = 7.7) and, with a high affinity for hydroxyl ions, above ph 3 has a tendency to form
insoluble Y(Oh)
3
species. Yttrium is redox stable in the 3+ oxidation state and is a hard metal ion with a preference for hard
donors such as carboxylate-oxygens and amine-nitrogens.
90
Y is a β
-
emitter used for therapy, and although it is possible to
perform biodistribution, imaging, and dosimetry studies with its bremsstrahlung X-rays, the spatial resolution obtained is
poor [48].
90
Y also emits a very low abundance of positrons (0.003%), which can be used to collect PET imaging data that
are more accurate than those from
90
Y bremsstrahlung imaging [48]. Because
90
Y has no significant γ or β
+
emissions, radio-
pharmaceuticals that incorporate it require a 'matched isotope pair' to be used as a surrogate for imaging and dosimetry.
Yttrium has been most commonly investigated as its therapeutic isotope
90
Y; however,
86
Y offers an attractive PET imaging
isotope that shares identical chemistry and therefore can be seamlessly substituted for
90
Y into existing radiopharmaceutical
preparations as a 'matched isotope pair' [1-6].
86
Y is a cyclotron-produced isotope that has a significant positron abundance