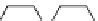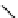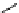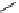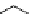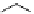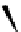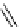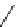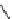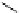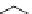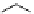Chemistry Reference
In-Depth Information
of 33% and a half-life of 14.7 hours (Table 5.1) that in comparison to a 64.2-hour half-life for
90
Y is relatively short.
111
It has
a closer matched half-life of 67.2 hours and is most typically used as a sPECT imaging surrogate ('matched isotope pair')
for performing dosimetry with
90
Y-based therapeutics. despite this mismatch in half-life,
86
Y is chemically identical to
90
Y
and is therefore a biologically equivalent surrogate, in contrast to
111
In, which can have completely different properties in its
complexes and their biodistributions [1-6].
86
Y is also a PET isotope, which is generally preferred to sPECT in terms of
spatial resolution and quantitative dosimetry [48].
86
Y has a relatively high-energy positron emission of 1221 keV, and along
with its low branching ratio of 33% and high-energy γ emissions, it is less ideal for PET imaging than are other long-lived
alternatives such as
89
Zr and
64
Cu, with their more favourable emission profiles.
86
Y is typically cyclotron-produced via the
nuclear reaction
86
sr(p,n)
86
Y, and purified by ion exchange chromatography or electrolysis [49, 50].
5.4.1
clinical trials Based on
86
yttrium
The long half-lives of
86/90
Y match well with the long biological half-lives of antibodies, which take two to three days to fully
penetrate tumours and can remain circulating
in vivo
for weeks [26]. Antibodies are typically metabolised in the liver, which
will subject conjugated radiometal-chelate complexes to harsh conditions [26]. Antibody conjugates with long circulation times
and prominent hepatobiliary metabolism make the need for high thermodynamic and kinetic stability even more important than
for conjugates of relatively fast clearing small biomolecules such as peptides (i.e., RGd, octreotide) [26]. unlike magnetic res-
onance imaging (MRI), contrast agents (Chapter 8) that utilise an open coordination site for water exchange, it is best that the
coordination sphere of the quasi-lanthanide Y be fully saturated for maximum stability when used in radiopharmaceuticals.
There is currently one Y-based radiopharmaceutical FdA-approved for clinical use,
90
Y-ibritumomab tiuxetan, trade name
Zevalin
®
[51].
90
Y-Zevalin is a radioimmunotherapeutic (RIT) for non-hodgkins lymphoma that utilises the BFC tiuxetan
conjugated to the antibody ibritumomab [51]. Tiuxetan is a modified version of the chelator dTPA, whose carbon backbone
contains an isothiocyanatobenzyl and a methyl group (Figure 5.4). Zevalin is also approved for use with
111
In for imaging and
dosimetry applications [52]. Zevalin could be used with
86
Y for PET imaging/dosimetry, because different isotopes of the same
element have identical chemical properties, but
86
Y-Zevalin is currently not approved [1-6]. The success of Zevalin in the
clinic for treating non-hodgkins lymphoma is well established [52]. Clinical trials are currently underway to investigate the
co-administration of Zevalin with the non-radiolabelled antibody rituximab to potentially enhance treatment success [53].
There are several Y-based radiopharmaceuticals in clinical trials; however, none of them utilise the positron-emitting iso-
tope
86
Y. As previously mentioned, the chemistry of
86
Y and
90
Y are identical (with the exception of possible differences in
specific activity and concentration), so all
90
Y-based therapeutics could potentially be used for
86
Y PET imaging agents,
should the properties of a given radiopharmaceutical agent warrant its use in PET imaging (i.e., for dosimetry as a theranos-
tic pair) [1-6]. To this end,
90
Y-based radiopharmaceutical agents that are currently in clinical trials will be discussed.
Although BFC systems are most common, nanoparticles are gaining traction in the literature, and glass microspheres (i.e.,
Theraspheres) that incorporate
90
Y are currently in clinical trials [54]. They are injected into arteries that flow into liver
tumours, where they become lodged in capillaries and bombard liver cancer cells with high doses of localised radiation [54].
ibritumomab
S
HN
HN
HO
2
C
N
N
N
CO
2
H
N
N
N
CO
2
H
HO
2
C
HO
2
C
HO
2
C
CO
2
H
CO
2
H
CO
2
H
CO
2
H
ibritumomab tiuxetan
DTPA
SCN
N
N
N
CO
2
H
HO
2
C
HO
2
C
CO
2
H
CO
2
H
p
-SCN-DTPA
fIgure 5.4
Commonly used Y(III) chelator dTPA, the BFC precursor
p
-sCn-dTPA, and the FdA-approved BFC system ibritu-
momab tiuxetan (trade name Zevalin when used with
90
Y).