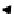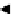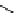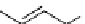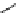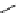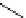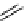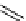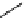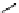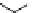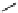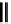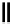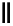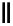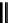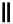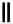Chemistry Reference
In-Depth Information
O
NH
2
HO
OH
HO
OH
glutamate-
pyruvate
transaminase
O
O
O
O
*
*
NH
2
NH
2
NH
2
ATP, Mg
2+
ADP
NAD
+
NADH
*
NH
2
OH
HO
OH
OH
13
NH
3
glutamate
dehydrogenase
O
O
O
O
O
glutamine synthase
[
13
N]L-glutamine
[
13
N]L-glutamate
[
13
N]L-alanine
O
OH
aspartase
HO
O
*
O
NH
2
OH
HO
O
[
13
N]L-aspartate
scheme 4.26
summary of the enzymatic synthesis of N-13 labelled amino acids.
1.K
2
[PtI
4
]
2. AgCl
cis-[PtCl
2
(13/14
NH
3
)
2
]
13/14
NH
3
scheme 4.27
synthesis of N-13 labelled cisplatin.
*
O
NH
2
O
Cl
13
NH
3
THF/Na
2
CO
3
O
O
O
2
N
O
2
N
[
13
N]NPC
scheme 4.28
synthesis of N-13 labelled NPC.
4.3.3
synthesis of [
13
n]cisplatin
Cisplatin,
cis
-[PtCl
2
(NH
3
)
2
], has been used for the treatment of a range of cancers, including brain tumours. N-13 labelling
of the widely used anti-cancer treatment cisplatin has been achieved using
13
NH
3
in order to better understand its blood-
brain-barrier penetration for improving brain tumour treatment. N-13 labelled cisplatin was prepared by the reaction of
K
2
Pti
4
with [
13
N]ammonia, forming
cis
-[Pti
2
(
13/14
NH
3
)
2
] [152, 153], following ligand exchange of iodide to chloride; using
silver chloride,
cis
-[PtCl
2
(
13/14
NH
3
)
2
] was obtained in good rCy (scheme 4.27).
Holschbach et al. [154] reported a more refined synthesis using solid phase extraction (sPE) technology. A strong anion
exchange (sAx) cartridge was used for the [
13
N]ammonia introduction step, and a cation exchange (sCx) cartridge was used
to remove cationic silver species after ligand exchange. A decay-corrected rCy of 80% was obtained with 30 MBq/mmol
specific activity.
4.3.4
synthesis of [
13
n] carbamates and ureas
The preparation of the N-13 labelled compounds performed using carrier-added methods results in specific radioactivities
that are much lower than the carrier-free methods typically used for
11
C and
18
F labelling. High specific radioactivities of
labelled compounds are generally required for brain-receptor studies in order to avoid saturating the receptor sites with cold
compound. No-carrier-added production methods are therefore essential for N-13 if it is to be used for these types of studies
in PET. An example of a no-carrier-added reaction for the production of a high specific activity compound is the synthesis
of
p
-nitrophenyl [
13
N]carbamate ([
13
N]NPC) from [
13
N]ammonia and
p
-nitrophenyl chloroformate [145] (scheme 4.28).
The high reactivity of chloroformate precursor was key to the success of this reaction; however, chloroformate analogues
were found to be difficult to handle owing to their high sensitivity to moisture.