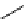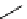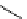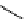Chemistry Reference
In-Depth Information
16
O(p,
α
)
13
N
AG50W-X8
13
NH
3
anhydrous
13
NH
3
aq.
10 mM EtOH
elution with 2N KOH
dessication by CaO
scheme 4.24
in-target production route of
13
NH
3
.
O
LiAlH
4
*
*
R H
2
O
R
H
2
Ether
R
l
R=C
6
H
5
CH
2
, n-C
7
H
15
(C
10
H
21
)
3
B
O
*
C
10
H
21
NH
2
13
NH
3
NaOCl
0 °C
*
NH
2
NH
Al / HgCl
2
EtOH
scheme 4.25
synthesis of N-13 amines from
13
NH
3
.
Although the in-target
13
NH
3
production generating higher specific radioactivities for PET imaging is a more recent
development, N-13 labelling chemistry using [
13
N]ammonia has been studied since the 1970s using low specific activity carrier
added methods. The short 10-minute half-life of nitrogen-13 has clearly limited the range of N-13 chemistry with the majority
of N-13 chemistry being dominated by
13
NH
3
production for myocardial blood flow measurements [128, 135-137]. Despite
the obvious time challenges, N-13 labelling routes have been developed for amino acid synthesis using enzymes [138-143],
acid chloride substitution reactions for amide synthesis [139, 144], and nitrophenyl carbamate synthesis (scheme 4.25) [145].
4.3.1
synthesis of
13
n-labelled amines
N-13 labelled amines are of interest for improving PET myocardial perfusion imaging and representing their metabolism.
The preparation of N-13 labelled amines has been achieved in good radiochemical yields via the reduction of N-13 amides
using LiAlH
4
, for example, [
13
N]phenetylamine and [
13
N]octylamine have been prepared from their respective N-13 amides
in excellent rCys (60-70%) [146]. The synthesis of [
13
N]phenetylamine via Hoffman rearrangement of the corresponding
amide is also possible, but rCys were poor owing to the suspected decomposition of the product by excess of NaOBr in the
reaction mixture [147]. The rCy was improved by using LiAlH
4
but a longer reaction time (5-20 min.) and tedious work-up
were unsuitable for the routine N-13 labelling. The synthesis of [
13
N]amphetamine was accomplished by the reduction of the
corresponding imine prepared from phenylacetone and [
13
N]ammonia [148]. in this process, a mixture of aluminium and
HgCl
2
was used as the reductant. However, carrier ammonia is required to avoid the formation of secondary amine, which
results in significantly decreased specific radioactivity. Organoborane precursors have recently been used for the preparation
of N-13 labelled organic amines. The amination reaction of tri-decylborane with [
13
N]ammonia in the presence of NaOCl
resulted in the rapid formation of [1-
13
N]aminodecane in 40-60% rCy (Figure 4-23) [149].
4.3.2
enzymatic synthesis of
13
n-labelled amino acid
radiolabelled amino acids are attractive molecules for PET studies because of their role in better understanding myocardium
and tumour metabolism. The labelling of an α-amino group or amide group with N-13 has been achieved using enzymatic
reactions. A key step in this labelling process was the immobilisation of enzymes onto a surface in order to avoid contamination
of the final labelled product [150]. Enzymes such as L-glutamic acid dehydrogenase, L-glutamine synthetase, L-glutamic-
pyruvic acid transaminase, and L-aspartase were immobilised to CNBr-activated sepharose [140] and used for the enzymatic
reaction of
13
NH
3
with amino acid precursors. The synthesis of
13
N-labelled L-glutamate was achieved via the reductive
amination with α-ketoglutarate and
13
NH
3
while the N-13 labelled L-glutamine was obtained by the amidation of L-glutamate
with
13
NH
3
. Nitrogen-13 labelled L-aspartate was prepared by fumarate amination. reactions are summarised in scheme 4.26
and were performed under the no-carrier-added conditions using a semi-automated procedure [140, 142, 150, 151].